Abstract:
In recent years, several technologies have been developed for modifying cotton and cotton blends as multi-functional textiles. Surface modification of cotton fabrics can impart wrinkle free finishes, self-cleaning properties, anti-microbial activity, UV protection, and flame retardancy. Self-cleaning features include Chitosan finish.
Chitosan is polysaccharide polymer containing more than 5,000 glucosamine and acetyglucosamine units, respectively. And their molecular weights are over one million Dalton’s. Chitin is found in fungi, arthropods and marine invertebrates. Commercially, chitin is derived from the exoskeletons of crustaceans (shrimp, crab and other shellfish) Chitosan is obtained from chitin by a deacetylation process.
Chitin, the polysaccharide polymer from which chitosan is derived, is a cellulose-like polymer consisting mainly of unbranched chains of N-acetyl-D-glucosamine. Commercial chitosan is derived from the shells of shrimp deacetylated chitin, or chitosan, is comprised of chains of D-glucosamine.
Chitosan can also be used in water processing as a part of a filtration process .Chitosan causes the fine sediment particles to bind together and is subsequently removed with the sediment during sand filtration .Chitosan also removes phosphorous, heavy minerals, and oils from the water .Chitosan is an important additive in the filtration process .sand filtration apparently can remove up to 50% of the turbidity alone while the Chitosan with sand filtration removes up to 99% turbidity.
1. Introduction:
Cotton is most important cellulose fiber in textile. But natural cotton contains some impurities. Many finishes are applied on these cotton and cotton blends. Special finishes like stain repellency finish is also applied on cotton to make it Antimicrobial finish. Teaching needs to be regularly upgraded and improved to meet the requirements of the industry and reflect the concerns of the society regarding health and environment. This includes knowledge of latest research and innovations covering the entire range from fibre to fabric manufacturing techniques and then fabric to three dimensional finished garments.
The inherent properties of the textile fibres provide room for the growth of micro-organisms. Besides, the structure of the substrates and the chemical processes may induce the growth of microbes. Humid and warm environment still aggravate the problem. Infestation by microbes cause cross infection by pathogens and develop odour where the fabric is worn next to skin. In addition, the staining and loss of the performance properties of textile substrates are the results of microbial attack. Basically, with a view to protect the wearer and the textile substrate itself antimicrobial finish is applied to the textile materials.
Antimicrobial textile products continue to increase in popularities demand for fresh smelling, skin friendly, and high-performance fabrics. Modern performance fabrics are required in much specialist application, sports textile is one example. This need to exhibit high degrees of performance in terms of longevity and durability by imparting antimicrobial properties to the fabrics. These properties can be improved as well as increase the comforts as hygiene factor making them more pleasant to wear. Odour can be neutralized and skin problems caused by microbial growth reduced thus emphasizing the ‘hygienic’ nature of the treated product.
Microbes are the tiniest creatures not seen by the naked eye. They include a variety of micro-organisms like Bacteria, Fungi, Algae and viruses. Bacteria are unicellular organisms which grow very rapidly under warmth and moisture. Further, sub divisions in the bacteria family are Gram positive (Staphylococcus aureus), Gram negative (E-Coli), spore bearing or non-spore bearing type. Some specific types of bacteria are pathogenic and cause cross infection. Fungi, molds or mildew are complex organisms with slow growth rate. They stain the fabric and deteriorate the performance properties of the fabrics.
Growing awareness towards health and hygiene has increased the demand of bioactive textiles. A durable finish is potentially effective means of controlling micro-organism on to textiles. In last few decades, wide varieties of antimicrobial agents have been used for the protection of textile as well as wearer. The major class of antimicrobial agent for textiles includes triclosan, metal and their salts, organo metallics, phenols, quaternary ammonium compounds, organometallics, phenols, quaternary ammonium compounds and organosilicons. One prime consideration related to the end use and function of an antimicrobial finish on textiles is the low toxicity of the finishing agent and such chemical finishes applied to textiles should meet environmental and low toxicity criteria. The use of natural antimicrobial agents on textiles dates back to antiquity, when the ancient Egyptians used species and herbs to preserve mummy wraps. Chitosan are the richest source of antimicrobial compounds.
2. Materials and Methods
- MATERIALS: – The plain-woven 100%cotton fabric will used for the study.
Table 2.1: – Fabric particular
| Sr. No | Particular | 100 % Cotton |
| 1 | Material | 100% cotton fabric |
| 2 | Weave | Plane |
| 3 | GSM | 150 |
| 4 | EPI | 70 |
| 5 | PPI | 70 |
| 6 | Warp count | 22 |
| 7 | Weft count | 22 |
2.2 Chemical: –
Table 2.2 Chemicals Used for Study
| Sr. No | Name of Chemicals | Purpose |
| 1 | Enzyme Amylase | Desizing Agent |
| 2 | Sodium Bicarbonate | To adjust pH |
| 3 | Sodium Hydroxide | Scouring Agent |
| 4 | Turkish Red Oil | Wetting agent |
| 5 | Hydrogen Peroxide (30%) | Bleaching Agent |
| 6 | Sodium meta Silicate | Stabilizer |
| 7 | Sodium Hexa Meta Phosphate | Sequestering Agent |
| 8 | Chitosan | Antimicrobial Agent |
| 9 | Citric Acid | Auxiliaries |
| 10 | Acetic Acid | To Adjust pH |
2.2 Experimental Method: –
2.2.1 Desizing :- 100 % cotton fabric treated with 3-5 gpl cellulase enzyme desizing to remove size paste. Desizing of cotton fiber was carried as follows:
Table 2.3: – Chemicals used for desizing
| Sr. No | Chemical | Quantity |
| 1 | Cellulase | 3-5 gpl |
| 2 | Wetting agent | 1 gpl |
| 3 | Sequestering agent | 1 gpl |
| 4 | Temperature | 60-70°C |
| 5 | Time | 90 minutes |
2.2.2 Combine Scouring and Bleaching
In combined scouring and bleaching of cotton, the scouring process is accelerated in presence of H2O2 and less time is required to achieve good absorbency of the material. The process of combined scouring and bleaching was carried using Hydrogen peroxide (H2O2) and Sodium Hydroxide (NaOH). After Completion of this process washing and draining of fabric was carried out. The process of combine scouring and bleaching is carried out in alkaline pH which is maintained by Sodium Carbonate (Na2CO3). pH of combine scouring and bleaching is 9-11. The combine scouring and bleaching was carried out as follows:
Table 2.4: – Chemicals used for Combine scouring and bleaching
| Sr. No | Chemical | Quantity |
| 1 | H2O2 | 3-5% {owf} |
| 2 | Sodium hydroxide | 3% {owf} |
| 3 | Sodium Carbonate | 1 gpl |
| 4 | Sodium Silicate | 1 gpl |
| 5 | Sequestering Agent | 1 gpl |
| 6 | Wetting Agent | 1 gpl |
| 7 | pH | 9-11 |
| 8 | Temperature | 85°C |
| 9 | Time | 90 minutes |
2.2.3.Preparation of chitosan chemical and application: –
Chitosan was dissolved in 2% aqueous acetic acid solution. The fabric was first immersed in the pad bath for 10 min. padded up to 80±5% wet pickup on weight of fiber [O.W.F.], dried on pin frames at 100ᵒC for 5 minutes. Cured at 180ᵒC for 2 minutes. washed and dried. Samples were cured at 180O for a period of 2 minutes.
2.2.4 Treatment of Chitosan on Fabric
The pre-scoured and bleached cotton fabric will pad-dry-cure with different concentrations of solution. Were prepared by dissolving chitosan overnight at Room Temperature. Containing various concentration of Chitosan (3gpl, 4gpl, 5gpl, 6gpl, 7gpl, 8gpl, 9gpl 10gpl, 11gpl, 12gpl, 13gpl, 14gpl, 15gpl) keeping 65% expression. The padded fabric samples were then dried at 80-85OC to maintain the residual moisture content 8–10%. The dried fabric
Table 2.5: – Concentration of chitosan
| Sr. No | Concentrations in GPL | Temperature in °C |
| 1 | 3 | 180°C |
| 2 | 4 | 180°C |
| 3 | 5 | 180°C |
| 4 | 6 | 180°C |
| 5 | 7 | 180°C |
| 6 | 8 | 180°C |
| 7 | 9 | 180°C |
| 8 | 10 | 180°C |
| 9 | 11 | 180°C |
| 10 | 12 | 180°C |
| 11 | 13 | 180°C |
| 12 | 14 | 180°C |
| 13 | 15 | 180°C |
2.3 Testing and Analysis
2.3.1 Antimicrobial Activity
Anti-microbial testing was done by AATCC test method 100:2004 for the quantitative assessment of the antibacterial effectiveness of the antimicrobial agents against Gram positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli). Circular swatches of 4.8 ± 0.1 cm in diameter were cut from the test fabric. The cut pieces were stacked in 250 ml wide mouth glass jar with a screw cap followed by sterilization at 121 °C for 15 min. 0.5 ml of the bacterial solution was added to the swatches so that whole of it is absorbed by one swatch. The jar was kept for 24 h in the incubator at 37 °C. After 24 h 50 ml of sterilized saline water was added to each jar followed by 15 min shaking in the shaker. Further three serial dilutions were done by taking 100 ml in 900 ml of saline water in eppendorf micro test tubes. Nutrient agar plates were made and 100 ml of this diluted bacterial solution was inoculated into the agar plate and left for 24 h in incubator at 37 °C. After 24 h the number of bacterial CFU of the bacteria formed on the agar plate were counted. Untreated cotton sample was used as the control sample every time.
2.3.2 Tensile Strength (ASTM D 5035)
Prior to the test specimens were conditioned to moisture equilibrium in the standard atmosphere of 65 % relative humidity, 27 ± 20C temperature. Samples (fabric strip) were cut by using the given template. Cut threads were removed from both side of the sample (raveling) to get strip of exactly 5cm width. Clamp was set on testing machine at distance of 20cm. and strength indicating pointer to zero position. Sample was clamped between two jaws, with some length of fabric extending beyond the jaws at each end. Sample was elongated at a constant rate of 300mm/min till a rupture. Breaking load in Kgf was noted. Same procedure ware repeated for all samples.
2.3.3 Measurement of Bending Length
Prior to the test specimens were conditioned to moisture equilibrium in the standard atmosphere of 65 % relative humidity, 27 ± 20C temperature. Samples (fabric strip) were cut by using the given template. Cut threads were removed from both side of the sample (raveling) to get strip of exactly 1-inch x 6-inch width. Put the sample on bending length track. Slide sample in forward direction till incline angle become 41.50. When fabric reach angle41.50 than measure the length on scale which is nothing but bending length
3. Result & discussion
3.1Antimicrobial activity
Table 3.1: Antimicrobial activity
| Sr.no | Concentration of Chitosan | Antimicrobial Activity (Percentage reduction in CFU) | |
| E. coli | S. aureus | ||
| 1 | 3 | 40.00 | 55.50 |
| 2 | 4 | 55.20 | 68.30 |
| 3 | 5 | 69.70 | 79.45 |
| 4 | 6 | 72.90 | 81.60 |
| 5 | 7 | 75.50 | 86.00 |
| 6 | 8 | 79.30 | 88.50 |
| 7 | 9 | 81.30 | 90.70 |
| 8 | 10 | 84.80 | 91.50 |
| 9 | 11 | 85.30 | 91.80 |
| 10 | 12 | 85.90 | 92.00 |
| 11 | 13 | 86.10 | 92.80 |
| 12 | 14 | 86.80 | 93.80 |
| 13 | 15 | 87.20 | 94.50 |

As shown in Fig .3.1, chitosan gives good anti-microbial activity when concentration increases from 3 to 10 gpl. With increase in concentration of chitosan antimicrobial activity increases. After 10 gpl there is no any remarkable improvement in antimicrobial activity. The maximum antimicrobial activity is archived at 15 gpl concentration which is 87.20 % for E. coli and 94.50 for s. aureus. The effect may increase with increase in concentration of chitosan but increase in concentration decrease the penetration of chitosan. With increase in concentration of solution and temperature of curing antimicrobial Activity increases. This is due to chitosan quantity increase.
3.2 Tensile strength
Table 3.2: – Tensile strength of given Treated fabric. (in kg/f)
| Sr.no | Concentration | Tensile strength (E. Coli) | Tensile strength (S. aureus) | ||
| Warp | Weft | Warp | Weft | ||
| 1 | Untreated | 22.30 | 22.25 | 22.30 | 22.25 |
| 2 | 3 | 21.95 | 21.90 | 22.00 | 21.95 |
| 3 | 4 | 21.95 | 21.90 | 22.00 | 21.95 |
| 4 | 5 | 21.80 | 21.85 | 21.90 | 21.80 |
| 5 | 6 | 21.80 | 21.80 | 21.85 | 21.80 |
| 6 | 7 | 21.80 | 21.75 | 21.80 | 21.80 |
| 7 | 8 | 21.75 | 21.65 | 21.75 | 21.70 |
| 8 | 9 | 21.75 | 21.55 | 21.70 | 21.60 |
| 9 | 10 | 21.65 | 21.45 | 21.65 | 21.50 |
| 10 | 11 | 21.60 | 21.35 | 21.60 | 21.40 |
| 11 | 12 | 21.50 | 21.30 | 21.60 | 21.40 |
| 12 | 13 | 21.50 | 21.30 | 21.50 | 21.30 |
| 13 | 14 | 21.45 | 21.25 | 21.50 | 21.30 |
| 14 | 15 | 21.45 | 21.20 | 21.50 | 21.25 |

Fig 3.2: – Tensile Strength
As shown fig 3.2, With increasing concentration of Chitosan, tensile strength decreases. But decrease in tensile strength is not remarkable. 2 – 3 % tensile strength decreases in both warp and weft direction. This is due to breaking of hydrogen bonds and decrease in air permeability of fabric.
3.3. Bending Length of given Treated fabric.
Table 3.3: – Blending length after application of chitosan
| Sr.no | Concentration | Tensile strength E. coli | Tensile strength (S. aureus) | ||
| Warp | Weft | Warp | Weft | ||
| 1 | Untreated | 2.50 | 2.50 | 2.50 | 2.50 |
| 2 | 3 | 2.80 | 2.85 | 2.85 | 2.85 |
| 3 | 4 | 2.80 | 2.85 | 2.85 | 2.90 |
| 4 | 5 | 2.95 | 2.90 | 2.98 | 2.95 |
| 5 | 6 | 2.95 | 2.95 | 2.98 | 2.95 |
| 6 | 7 | 2.95 | 3.00 | 3.00 | 3.10 |
| 7 | 8 | 3.00 | 3.00 | 3.10 | 3.10 |
| 8 | 9 | 3.05 | 3.05 | 3.10 | 3.10 |
| 9 | 10 | 3.10 | 3.05 | 3.20 | 3.25 |
| 10 | 11 | 3.20 | 3.05 | 3.30 | 3.25 |
| 11 | 12 | 3.20 | 3.10 | 3.30 | 3.30 |
| 12 | 13 | 3.25 | 3.10 | 3.40 | 3.30 |
| 13 | 14 | 3.25 | 3.15 | 3.40 | 3.40 |
| 14 | 15 | 3.20 | 3.15 | 3.50 | 3.40 |
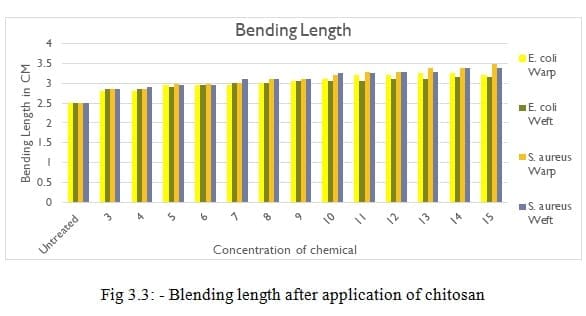
As shown fig 3.3, With increasing concentration of Chitosan, bending length also increases. 2 – 3 % increase in bending length observed in all concentration of chitosan. The increase in bending length is due to increase in stiffness of fabric.
4. Conclusion: –
In this study different concentration of antimicrobial agents were applied by pad-dry-cure technique and antimicrobial activity was evaluated against two bacteria i.e. S. aureus (Gram positive bacteria) and E. coli (Gram negative bacteria). The anti-microbial activity increases with increase in concentration of chitosan. When concentration increases from 3 to 10 gpl. With increase in concentration of chitosan antimicrobial activity increases. After 10 gpl there is no any remarkable improvement in antimicrobial activity. The maximum antimicrobial activity is archived at 15 gpl concentration which is 87.20 % for E. coli and 94.50 for s. aureus. There is no any remarkable change observed in physical properties of cotton fabric.
5. Reference
- Zitao Zhang, Liang Chen, Jinmin Ji, Yanliu Huang and Donghui Chen “Antibacterial Properties of Cotton Fabrics Treated with Chitosan” Textile Research Journal 2003 73: 1103
- Md Ibrahim H Mondal, Firoz Ahmed, Md Roknuzzaman, Md Nazmul Huda, Md Ahsan Habib “Antimicrobial activity of chitosan and its derivatives exhausted cotton fabrics as ecofriendly antimicrobial agents” Journal of Textile Eng Fashion Technol. 2020;6(3):77‒80
- Guneet Dhiman and J. N. Chakraborty “Antimicrobial performance of cotton finished with triclosan, silver and chitosan” Dhiman and Chakraborty Fashion and Textiles (2015) 2:13
- SEUNGSIN LEE, JEONG-SOOK CHO, AND GILSOO CHO “Antimicrobial and Blood Repellent Finishes for Cotton and Nonwoven Fabrics Based on Chitosan and Fluoropolymers” Textile Research Journal 1999 69: 104
- M. Parthiban, Dr. S. Gunasekaran & Silambarasan, Sakthi Srinivasan, Seetharaman, Karthika “Effect of nanosiver application on antimicrobial finishing”
- Thlagavathi, T.Kannaian, “Dual Antimicrobial and blood repellent finishes for cotton hospital fabrics”, IJFTR, volar, 33,2008,23-29
- DANIELA ENESCU “Use of Chitosan in Surface Modification of Textile Materials” Vol. 13, No. 6, 2008, pp. 4037-4048
- Allan, C. and Hadwiger, L.A. 1979 “The fungicidal effect of chitosan on fungi of varying cell wall composition”. Exp. Mycol., 3: 285–287. [Crossref], [Google Scholar]
- Boguslawski, S., Bunzeit, M. and Knorr, D. 1990. “Effects of chitosan treatment on clarity and microbial counts of apple juice”. ZFL, Int. J. Food Technol. Food Proc. Eng., 41: EFS55 [Google Scholar]
- Castellanos‐Perez, N., Maldanado‐Vega, M., Fernandez Villagomez G and Cafferal‐Mendez, S. 1988. “An evaluation of the coagulating ability of chitosans from different crustacea species and fungi”. In Chitin and Chitosan, Edited by: Skjak‐Braek, G., Anthonsen, T. and Sandford, P. 567–576. London: Elsevier Applied Science
- El-tahlawy, K.F., El-bendary, M.A., Elhendawy, A.G. & Hudson, S.M. (2005) “The antimicrobial activity of cotton fabrics treated with different crosslinking agents and chitosan” Carbohydrate Polymers, 60(4), 421–430.
Keywords: Chitosan, E. coli, S. aureus, antimicrobial.
Mrs. Supriya Shirhatti,
Mr. Satish Patil,
Department of textiles,
DKTES Textile & Engineering Institute Ichalkaranji-416115
Correspondence e-mail: –

