Properties of Si–C, Si–O, and Si–F bonds
In most organosilicon compounds, Si is tetravalent with tetrahedral molecular geometry. Carbon–Silicon bonds compared to Carbon–Carbon bonds are longer (186 pm vs. 154 pm) and weaker with bond dissociation energy 451 kJ/mol vs. 607 kJ/mol. The C–Si bond is somewhat polarised towards carbon due to carbon’s greater electronegativity (C 2.55 vs Si 1.90). The Si–C bond can be broken more readily than typical C–C bonds.
Si–O bonds are much stronger (809 kJ/mol compared to 538 kJ/mol) than a typical C–O single bond. The favorable formation of Si–O bonds drives many organic reactions such as the Brook rearrangement and Peterson olefination. The Si–O bond is even stronger than that of the Si–F bond, even though F is more electronegative than O.
Siloxanes generally adopt structures expected for linked tetrahedral (“sp3-like”) centers. The Si–O bond is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si–O–Si angle is rather open at 142.5°. By way of contrast, the C–O distance in a typical dialkyl ether is much shorter at 1.414(2) Å with a more acute C–O–C angle of 111°. It can be appreciated that the siloxanes would have low barriers for rotation about the Si–O bonds as a consequence of low steric hindrance. This geometric consideration is the basis of the useful properties of some siloxane-containing materials, such as their low glass transition temperatures.
Properties of Silicone
Bond angle:
- Si—O—Si bond angle 142.50 and varies from 1050 to 1800 while C-O-C bond angle 1110 is smaller and less variable.
- Si—O bond length is 1.64 A0 and Si—C bond is 1.92 A0
- Flexible backbone
- Low intermolecular attraction.
Bond Strength:
Minimal change in physical properties at wide temperature range
Si—O is 809 kJ/mol, C—C 348 kJ/mol, C—O bond energy 538 kJ/mol,Si—O—Si bond strength is stronger than C—C and C—O bond strength.
Freely Rotating Methyl Groups:
- Keep chains separated (large free volume)
- Electrostatic attraction decreases with distance
Low Glass Transition Temperature (Tg):
- Glass Transition Temperature of Silicone is -1200C
- Remains pourable at very low temperature
- Very low brittleness
Low Surface Energy:
- Methyl group can swing to surface
- Inherent water repellency property
- Defoaming and antifoaming characteristics
- Slippery in nature
Minimal changes in physical properties with temperature:
- It is due to low Glass Transition temperature ( -1200C)
- Very high bond strength of Si—O—Si (108 Kcal/mole)
Because of these inherent properties of Silicone, it possesses some significant features.
- Silicones are Oxidation resistant
- Temperature resistant
- Ozone resistant
- Moisture resistant
- Vapor permeability
- Bio- compatibility
- Insulators
- Flexible and elastomeric in nature
- Antifoam property
Functional groups
Silicon is a component of many functional groups
Silanols, Silyl ethers, Silyl chlorides, Epoxy, Carboxy, Amino
Silanols are analogues of alcohols. They are generally prepared by hydrolysis of silyl chlorides:
R3SiCl + H2O → R3SiOH + HCl
Silyl ethers:
Silyl ethers have the connectivity Si-O-C. They are typically prepared by the reaction of alcohols with silyl chlorides:
(CH3)3SiCl + ROH → (CH3)3Si-O-R + HC
Silyl chlorides:
Organosilyl chlorides are important commodity chemicals. They are mainly used to produce silicone polymers. Especially important silyl chlorides are dimethyldichlorosilane (Me2SiCl2), methyltrichlorosilane (MeSiCl3), and trimethylsilyl chloride (Me3SiCl). More specialized derivatives that find commercial applications include dichloromethylphenylsilane, trichloro(chloromethyl)silane, trichloro(dichlorophenyl)silane, trichloroethylsilane, and phenyltrichlorosilane.
Silyl hydrides:
The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon hence the naming convention of silyl hydrides. Commonly the presence of the hydride is not mentioned in the name of the compound. Triethylsilane has the formula Et3SiH. Phenylsilane is PhSiH3. The parent compound SiH4 is called silane.
Epoxy functional group:
Epoxy group is incorporated into Silicone backbone through hydrosilylation reaction with silyl hydride and allyl glycidyl ether.
(Cl)2CH3Si—-H + CH2 = CH-CH2-OCH2-CH–CH2 ——–Si—(CH2)3—O-CH2 CH—CH2
Amino functional group:
Amino group is incorporated into Silicone backbone through hydrosilylation reaction with silyl hydride and allyl chloride and then reaction with ethylene diamine.
(OMe)2-CH3–Si-(CH2)3-NH(CH2)2-NH2 MeOH reflux(Cl)2CH3Si—-H + CH2 = CH-CH2Cl Si-(CH2)3-Cl NH2-(CH2)2NH2
Basic manufacturing process of Chlorosilane in Fluidised Bed reactor
Absolute pure Silicon powder is used in the synthesis of methylchlorosilane in presence of Copper catalysts and promoters mixed to the contact mass. This Silicone powder is manufactured by Silica by charcoal reduction method. This contact mass is then introduced in the Fluidized bed reactor and reacted. Unreacted chloromethane, the gaseous Methylchlorosilanes, gaseous by-products, Catalyst components and finely divided dusts leave the Reactor.
SiO2 + C————Si + CO2
Si + CH3Cl Cu Catalyst ——- (CH3)2SiCl2 + CH3SiCl3 + (CH3)3SiCl + SiCl4
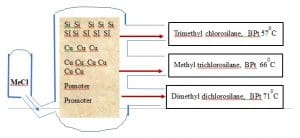
All these Chlorosilanes are separated by fractional distillation due to their different boiling points. After separating they are hydrolyzed to get various siloxane and from Dimethyldichlorosilane (CH3)2SiCl2 a key precursor to cyclic (D3, D4, etc.) and linear siloxanes.
Synthesis of siloxanes
Dimethyldichlorosilane (CH3)2SiCl2 is hydrolyzed and form a key precursor to cyclic (D3, D4, etc.) and linear siloxanes. Linear roduts are terminated by with Sillanol groups.
(CH3)2SiCl2 + H2O HOSi(CH3)2OH + HCl
By intermolecular and intramolecular H bonding forms cyclic (D3, D4, D5 etc.) and linear siloxanes.
Linear products are terminated by with Sillanol groups. The siloxane bond can then form via a silanol + silanol pathway or a silanol + chlorosilane pathway
n R2Si(OH)2 → H(R2SiO)nOH + (n−1) H2O
R3Si–OH + R3Si–Cl → R3Si–O–SiR3 + HCl
The linear products, polydimethylsiloxane (PDMS), are of great commercial value. Their production requires the production of dimethylsilicon dichloride
Cyclic products have no silanol terminal:
n R2Si(OH)2 → (R2SiO)n + n H2O R= CH3
D3 : Hexamethyl Cyclo trisiloxane
D4 : Octamethyl Cyclo tetrasiloxane
D5 : Decamethyl Cyclo pentasiloxane
Trimethyl Chlorosilane(TMCS) after hydrolysis forms hexamethyl disiloxane and used as chain terminating agent to control molecular weight of Silicone polymer.
2 R3Si–Cl + H2O → R3Si–O–SiR3 + 2 HCl
2 R3Si–OH → R3Si–O–SiR3 + H2O
Methyl trichlorosilane (MTCS) after hydrolysis gives three dimensional Silicone resin based product.
2 RSi–Cl3 + H2O → RSi–(OH)3 + 3 HCl
OH OH
RSi–(OH)3 + RSi–(OH) 3 → RSi—-O——SiR + H2O
OH OH
Silicone tetrachloride after hydrolysis
Si(Cl)4 + H2O → Si(OH)4
O O
Si(OH)4 + Si(OH)4 → O Si——O——–Si O
O O
Polymerisation of Cyclic (D3, D4, D5..) to make Silicone Polymer :
Ring Opening Polymerisation :
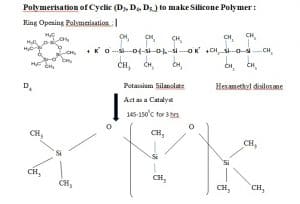
Ring opening polymerization occurs through addition polymerisation in three different stages.
Stage 1 : Initiation
In this stage Potassium Silanolate catalyst opens the cyclic ring of D4 and generates initiator.
Stage 2 : Propagation
In this stage Initiator reacts no of moles of Cyclic D4 and propagate the chain and molecular weight increases.
Stage 3 : Termination
In this stage terminal (O–) of propagating chain of both the sides react with hexamethyl disiloxane, chain terminator and stops the polymerization and desired molecular weight is obtained.
Classification of Silicones
Silicones are classified into two broad category. One is Inorganic Silicone and the second one is Organic Silicone.
Inorganic Silicone :
Inorganic Silicone is a highly active adsorption material usually a reaction with Sodium Silicate and Sulphuric acid and aging and its chemical formula is mSiO2, nH2O. It is insoluble in water and any solvent, non toxic, tasteless, chemically stable, in addition to alkali it does not react with hydrofluoric acid also. It has high adsorption property, excellent thermal stability and very high mechanical strength. It is used in various Industries, such as the common colorful kids slap watches are made of Inorganic Silica.
Organic Silicone :
Organic Silicone is an organic Silicone compound refers to those containing a Si-C bond, and at least one organic group is directly connected to Silicone atom in the compound, it is customery also oftenes to the organic group and the Silicone through Oxygen, Sulfur, Nitrogen, etc atoms connected to the compound is also used as Organic Silicone compound.
Wherein the Silicone Oxygen bond ( –Si—O—–Si–) as backbone consisting of a Polysiloxane which is more than 90% of the total amount of organic Silicone compound.
It is used in various Industries including Textile, Fiber Finish, Personal care, Health Care, Rubber both high temperature vulcanization (HTV) and Room temperature vulcanization (RTV), Automobile, Eletronic, Space research and so on.
This article will exclusively focus only on Textile and fiber finish segment.
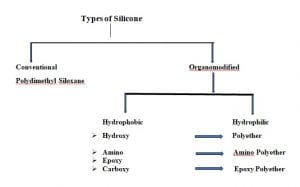
Types of Organic Silicone
Organic Silicones are broadly two types. One is Conventional Silicone and the second one is Organomodified Silicone. Classic example of Conventional Silicone is Polydimethy Silicone.
Depending on the functional group present, Organomodified Silicone is further classified into two type. One is Hydrophobic and the other is Hydrophilic.
Hydrophobic Silicone includes Hydroxy, Amino, Epoxy and Carboxy group.
Hydrophilic Silicone includes Polyether, Amino Polyether and Epoxy Polyether.
Application area in Textile vis a vis structure of Silicone molecule
Application Area 1
Oldest Silicone used in Textile is named Polydimethyl Siloxane (PDMS).
PDMS is inert, does not contain any functional group and has different viscosity having different molecular weight. It is used either in oil form with suitable ecofriendly solvent or in oil in water emulsion discussed later.
Application of PDMS Silicone oil:
As a non aqueous based Sewing thread lubricant for Polyester Yarn in Kiss Roll application
Application of PDMS emulsion:
1. Sewing thread lubricant for Polyester Yarn by Exhaust application with Polyethylene emulsion.
2. Softener for dry feel application
3. Defoaming and antifoaming agent with hydrophobic Silica
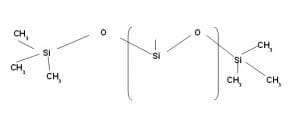
Polydimethyl Siloxane (PDMS)
Application area 2
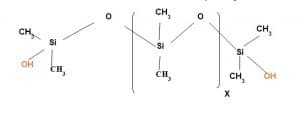
Wash durable hydrophobic lubricant:
It is hydroxy modified Polydimethyl Siloxane where one of the terminal Methyl group is replaced by hydroxy group. This hydroxy functional group acts as reactive group with cellulosic hydroxy group in presence of catalyst so that it shows wash durability property.
It is used in emulsion form.
Application of hydroxy modified PDMS emulsion:
1. Yarn lubricant
- Non yellowing Softener
Application area 3:
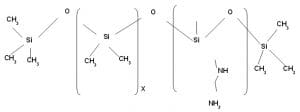
Wash durable hydrophobic Softener with high degree of softness
This is either micro or macro emulsion of Amino Silicone. Depending on the nature and concentration of emulsifier and type of amino Silicone micro or macro emulsion is formed.
Since it is an emulsion of Amino Silicone oil it has tendency to become yellowish on fabric due to the tendency to form Amine oxide by aerial oxidation of amine group attached to the Silicone back bone and if it is applied on dyed substrate shade variation is observed.
Application Area 4:
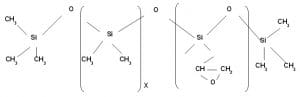
Reactive Epoxy modified wash durable hydrophobic Silicone Softener for dry feel application
It is used as a macroemulsion for textile application by padding method. Since it contains epoxy group it reacts with hydroxyl group present in cellulose and forms a covalent bond resulting durable finish.
Application area 5:
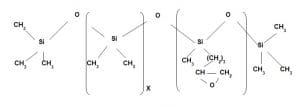
Wash durable hydrophobic bulky finish
This carboxy modified Polydimethyl silicone. It is used in the emulsion form. Since it contains carboxy functional group it reacts with hydroxyl group of cellulose in presence of catalyst at high temperature during curing and forms covalent bond as a result durable finish obtained. It is non yellowing and softness is less than amino Silicone. It has very less application in textile.
Application area 5:
Wash durable hydrophobic bulky finish
This carboxy modified Polydimethyl silicone. It is used in the emulsion form. Since it contains carboxy functional group it reacts with hydroxyl group of cellulose in presence of catalyst at high temperature during curing and forms covalent bond as a result durable finish obtained. It is non yellowing and softness is less than amino Silicone. It has very less application in textile.
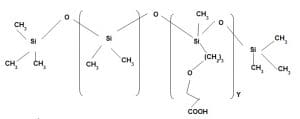
Application area 6:
Silicone based surfactant
It is Polyether based polydimethyl siloxane. It imparts wetting property. Depending on the polyether contents it acts as a hydrophilic agent also. It does not have softening property. It helps to improve wicking property so that it uses as a moisture management product also.
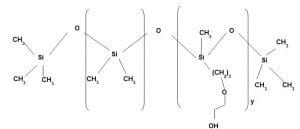
Application area 7:
Hydrophilic finish with very good softness
It is a Polyether based terpolymer of amino silicone having Polydimethyl Siloxane in other block. It imparts both hydrophilicity and softness. As the polyether content increases hydrophilicity increases but at the same time softness decreases. Similarly with increase of amine value softness increases but yellowness also increases and shade variation is also noticed. To get the optimum result amine value and polyether content has to be balanced.
For Amino Silicone based textile softener amine value is generally maintained 8 to 30. For certain special cases amine value is maintained 40 but it is very rare.
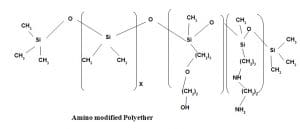
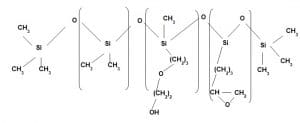
Application area 7:
Hydrophilic finish with dry and bouncy feel
It is a Polyether based terpolymer of epoxy silicone having Polydimethyl Siloxane in other block. It imparts both hydrophilicity and dry feel. As the polyether content increases hydrophilicity increases but at the same time softness decreases. Similarly with increase of epoxy content bouncyness increases.
Silicone Emulsion:
To discuss about Silicone emulsion let us first talk about emulsion.
The word “emulsion” comes from the Latin emulgere “to milk out,” from ex “out” + mulgere “to milk”, as milk is an emulsion of fat and water, along with other components.
An emulsion is a mixture of two or more liquids that are normally immiscible. one liquid called dispersed phase is dispersed in the other called continuous phase. Two liquids can form different types of emulsions. Oil and water can form, first, an oil-in-water emulsion, wherein the oil is the dispersed phase, and water is the continuous phase, example is milk where fat is dispersed phase and water is continuous phase. Second, they can form a water-in-oil emulsion, wherein water is the dispersed phase and oil is the continuous phase, Example is Butter where butter is continuous phase and the water is dispersed phase.
To make stable emulsion one important ingredient is used called emulsifier or surfactant. Emulsifiers contain both a water-loving head group and an oil-loving tail. When added to an o/w emulsion, the emulsifier surrounds the oil droplet with it’s oil-loving tail extending into the oil, and it’s water-loving head facing the water. (In a w/o emulsion, the emulsifier’s orientation is reversed.) In this way, emulsifiers lower the interfacial tension between the oil and water phases, and form a protective layer around the oil phase keeping the oil droplets evenly dispersed and preventing them from flocculating
Generally three types of emulsions are used in textile. One is Microemulsion, second one is macroemulsion and the third one in Nano-emulsion. All these emulsions are classified based on the particle size of dispersed phase. These particle sizes are governed by the nature and quantity of surfactant used to make emulsion. More the surfactant will be used less will be the particle size of dispersed phase and it will shift from Macroemulsion to Nano-emulsion.
The Silicone microemulsion is characterized as thermodynamically stable, isotropic mixtures of water, Silicone oil, and surfactant, particle size ranges from 100-150 nm.
Macroemulsions of Silicone are homogenous milky white thermodynamically unstable systems with particle sizes ranging from 150-300 nm. Macroemulsions scatter light effectively and therefore appear milky, because their droplets are greater than a wavelength of light.
The Nano emulsion of Silicone is characterized as thermodynamically stable, isotropic mixtures of water, oil, and surfactant, particle size ranges from 10-100 nm.
Silicone emulsions are broadly used in Textile as a softener. They are either emulsion of Polydimethyl Siloxane or emulsion of Amino Silicone. They are mainly Oil in Water emulsion.
In textile Polydimethyl Siloxane emulsion mainly used as defoaming and antifoaming agent along with hydrophobic or precipitated Silica. It is also used as sewing thread lubricant along with Polyethylene emulsion.
Amino Silicone emulsion is used more than 95% in textile as softener to improve hand feel.
It is used as Micro, Macro and Nano emulsion.
Classification of Silicone emulsion: Depending on the particle size, Silicone emulsion is also classified as Macro, Micro and Nano category and based on the water affinity it is classified as Hydrophobic and Hydrophilic type.
Silicone Microemulsion is used maximum in Textile industry as they are thermodynamically stable and gives desired hand feel. They are stable in hard water shear stability is good and also electrolyte stable. Since the particle size is small, 100-150 nm it can penetrate into fiber matrix and imparts both inner and outer softness.
Silicone Macroemulsion is used mainly is thermodynamically unstable. They are moderately stable in hard water shear stability and electrolyte stability are poor. Since the particle size is bigger >150 nm it cannot penetrate into fiber matrix and imparts only surface softness or smoothness.
Silicone Nano emulsion is extremely thermodynamically stable. They are very much stable in hard water, shear stability is very good and also electrolyte stable. Since the particle size is very small, 10-100 nm it can penetrate into fiber matrix mostly used to break the body and for moisture management application. It is explained in detail in Hydrophilic Silicone Part.
Hydrophobic Silicone emulsion: Silicone is inherently hydrophobic in nature due to the presence of Methyl group attached in the Silicon atom. Hydrophobic amino Silicone imparts very good softness but at the same time gives yellowness depending on the amine value. More the amine value more will be yellowing tendency. They may be Micro or macro emulsion.
Hydrophilic Silicone emulsion: Silicones are inherently hydrophobic in nature. The cloths and the garment treated with those Silicone softener feel discomfort when we wear particularly at humid condition. To transport the humidity to the atmosphere as fast as possible to evaporate it and making the skin dry Silicone has been made hydrophilic in nature. In the Silicone backbone polyether group is incorporated depending on the requirement. This hydrophilic Silicones are polyether based polydimethyl siloxane, polyether based epoxy silicone and polyether based amino silicone. As Silicone has very good spreading property these types of hydrophilic silicone has wonderful application in Textile as a Moisture Management product which is explained below.
Maintaining body heat under different environmental conditions is very essential for a fabric which determines its comfort level. A key performance criterion in this respect is the Moisture Management. The Moisture Management is defined as the “controlled movement of water vapour and liquid water (perspiration) from the surface of the skin to the atmosphere through the fabric” The human body releases around 60ml of water vapour at ambient conditions even when it is at rest. When we do some activity like walking or play any sports, the body warms up and sweats more which is more or less absorbed by the textile material. This humidity needs to be transferred to the surface of the fabric for evaporation and thus producing a cooling effect. Therefore, to make a wearer feel comfortable, not only should the fabric evaporate the perspiration from the skin surface to the fabric surface but, the moisture should also get evaporated. Moisture adds weight to the garment and makes the skin cold. It can also cause irritation and skin diseases. Hence, it is very essential to have a moisture management fabric so as to make the wearer feel comfortable.
Aim
The main aim of moisture management fabric is to make the skin feel dry. In order to achieve this, humidity should be evaporated and transferred to the atmosphere as soon as possible. The transportation of humidity to the surface of the fabric is done by a capillary force known as wicking. As the gaps between the individual fibres becomes thin the force increases. Thus, finer fibres will have smaller gaps and better humidity transport. The evaporation of the humidity depends on the surface area of the textile used. As the surface increases, fibres are finer and hence more fibres at the surface and faster humidity evaporation.
Requirements of a moisture management fabric:
For a fabric to be a good moisture management fabric it needs to fulfil the following attributes according to
- Optimum heat and moisture regulation
- Good air and water vapour permeability
- Rapid moisture absorption and conveyance capacity
- Absence of dampness
- Rapid drying to prevent catching cold
- Low water absorption of the layer of clothing just positioned to the skin
- Dimensionally stable even when wet
- Durable
- Breathability and comfort
- Easy care performance
- Lightweight
Moisture management route
Regarded as a second skin, the textile material should work synchronously and be compatible with the human body’s heat regulation mechanism. Transmission of the moisture in both liquid and vapour form is equally important to make the wearer feel comfortable. In this regard, wetting, wicking and moisture vapour transmission are of critical importance. This transmission occurs by three mechanisms.
- Simple diffusion through inter yarn spaces: Controlled by the water vapour pressure gradient across the inner and outer faces of the fabric, this is the main mechanism for transferring the vapour in low moisture content conditions. The size and concentration of inter yarn pores and the fabric thickness governs the resistance to diffusion.
- Capillary transfer through fibre bundles: Wicking of the liquid water through the yarns takes place which is then evaporated at the outer surface. The efficiency of yarn working depends on the surface tension, i.e., wet ability of the fibre surfaces, and the size, volume and number of capillary spaces is determined by the choice of yarn and fabric construction.
- Diffusion through individual fibres: Depending on the hydrophobic or hydrophilic nature of the fibres, the water vapour is absorbed into the fibres at the inner surface of the fabric, diffused through the fibre structure and desorbed at the outer surface.
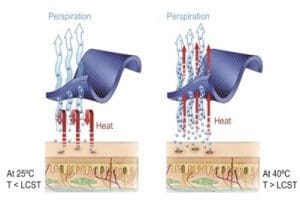
Applications of moisture management technique:
Following are the areas where the moisture management techniques are popularly applied:
- Inner wears
- Athletic wear (active sportswear)
- Performance wear (climbing, walking, skiing)
- Comfort wear (nightwear)
- Military (multi-climate clothing)
- Health (hospital bed linens, wound dressings)
- Agricultural technology (Geo-textiles, greenhouse screening panels, soil moisture control)
- Technical solutions (Formula 1 protective clothing, firefighting, industrial clothing)
- Industrial (filter & valve technology, building, packaging)
- Upholstery (transport)
Silicones recommended for Padding application:
Most emulsions we make are o/w which are generally, more stable and this is the type of emulsion we will be discussing in this article
For recommendation of any Silicone emulsion in Textile application following points to be considered.
- Stability of Silicone emulsion
- Application guideline
The process by which an emulsion coalesces (completely breaks i.e., the system separates into bulk oil and water phases), is generally considered to be governed by four different droplet loss mechanisms: Brownian flocculation, creaming, sedimentation flocculation, and disproportionation
To check Emulsion stability for both micro and macro emulsion, following test has to be conducted.
- Shear stability: by centrifuge method, at 3000rpm for 30 min, no oil should be on top
- Freeze Thaw stability: product has to be kept at 500C for 8 hours and the same emulsion to be kept for 8 hours at 40 This is called 1 cycle. In this way minimum 8 cycles has to be passed.
- Heat accelerator Test: Taken 2% emulsion into stoppered test tube and put into Beaker containing water at 700C for 2 hours. No silicone layer should observe on top
- Hard water Test: 2% emulsion is made at 500-800 ppm Hard water and kept for 8 hours. Observed change of appearance.
For Application guideline following care has to be taken.
- pH of the Bath: pH of the Bath has to be 4.5 to 6.5. After running few hundred meter
fabric core alkali comes out and made the bath alkaline and that time again bath has
to be maintained acidic at that range.
- Hardness of Water: Silicone emulsions are not stable in hard water. When we prepare bath at particular concentration by using water care should be taken that water should be soft. Hardness of water should not be above 10 ppm.
Silicones recommended for Exhaust application:
To recommend Silicone emulsion for Exhaust application extreme care has to be taken in terms of Type of Silicone Emulsion is being recommended, Stability of Emulsion and Application guideline.
Type of Silicone Emulsion:
- Only micro and nano emulsion having hydrophilic in nature can be recommended
- Block Silicone is recommended for Exhaust application
Stability of Emulsion:
- All the stabilities discussed above should be passed
- Additional Shear stability test by Foam Rig Instrument.
Application guideline:
-
- pH of the bath should be 4.5 to 6.5
- Hardness of the water should be below 10 ppm
- Temperature of the bath should be below 450C
- Time for exhaustion should be 20- 30 min
Problem faced by Silicone Softeners during application:
- Silicone Spot on Fabric: Silicone Spot is a very important terminology in Textile Industry. During application of Silicone Softener which are basically in emulsion form prone to break if any of the above discussed parameters of emulsion stability is not checked carefully. If the application guideline is not followed properly Silicone emulsion may break and Silicone spot will appear on fabric. During wet condition this Silicone sot is not observed. After passing through stenter this spot appears and no chemical is invented so far to remove this spot.
- Deposition of Silicone on Padding mangle: Silicone deposition on Roller Bed is noticed during padding application after running few hundred meters of fabric. This is also the breakage of Silicone due to poor shear stability of Silicone emulsion and not followed proper application guideline.
Summarization of uses of Silicone in Textile application
- In the defoaming and antifoaming application: Wetting agent and detergent are the primary Textile auxiliary used in Scouring and bleaching application. To make it low foaming either Silicone emulsion or 100% ethoxylated and propoxylated based Polydimethyl Siloxane are used in the formulation.
- Silicone based surfactant: In the fiber finish application particularly for man made fiber, like Polypropylene and Polyester which are hydrophobic in nature polyether based Silicone surfactant is used as additive to improve the wetting property.
- Silicone Softener: More than 95% Textile application Silicones are used as a softening agent to make the substrate very smooth, soft and silk and elastomeric and bouncy. Mostly Amino Silicones and polyether based amino Silicone to get hydrophilic property are used for softening application.
- Sewing thread lubricant: For Sewing thread lubricant polydimethyl siloxane either in oil form or in emulsion form are used. Application is either in Kiss roll application where 100% PDMS oil is used or in exhaust application where PDMS emulsion is used.
- Colour blooming agent: Silicone has an inherent property to enhance the color strength of dyed substrate particularly disperse dye for black, Red and navy blue.

