Abstract
Some substances, Dyestuffs or pigments or any chemicals appears homogenous, but are actually a combination of substances of different mixtures of dyes, chemicals, or different compounds. In addition, the many dye are used in this dyestuff area is a mixture of different colored materials. In many instances, we can separate these materials by dissolving them in an appropriate liquid and allowing them to move through an absorbent matrix, like paper, liquid, Thin-Layer Chromatography (TLC), etc. Chromatography is a method used in laboratory for separating organic and inorganic compounds so that they can be analyzed and studied. By analyzing a compound, a scientist can figure out what makes up that compound. Chromatography is a great physical method for observing mixtures and solvents.
Chromatography is a powerful separation tool that is used in all branches of science, and is often the only means of separating components from complex mixtures. The Russian botanist Mikhail Tswett coined the term chromatography in 1906. The first analytical use of chromatography was described by James and Martin in 1952, for the use of gas chromatography for the analysis of fatty acid mixtures.
Keywords: Dyes, Chromatography, TLC, HPLC, Paper-Gas-Liquid-Chromatography.
Introduction Chromatography is a aboratory technique for the separation of a mixture of color or pigments, chemicals. The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. The various constituents of the mixture travel at different speeds, causing them to separate. The separation is based on differential partitioning between the mobile and stationary phases. Subtle differences in a compound’s partition coefficient result in differential retention on the stationary phase and thus affect the separation. (01)
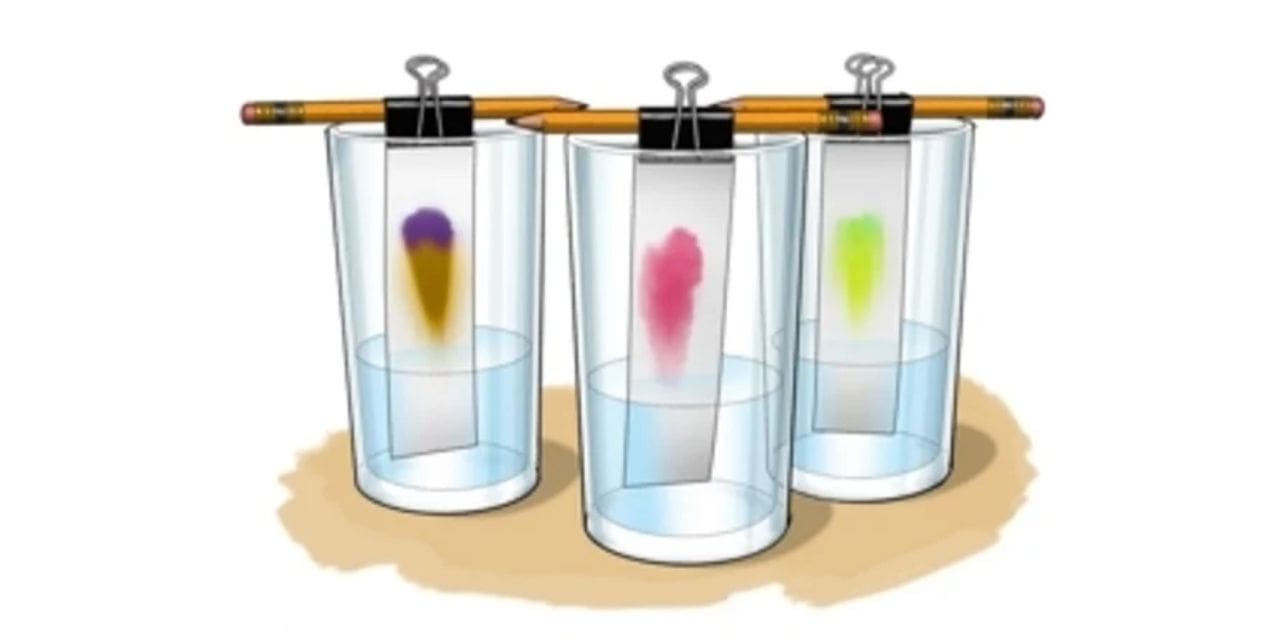
Some of the colored substances dissolve in the solvent used better than others, so they travel further up the paper. A pencil line is drawn, and spots of ink, pigment or dye are placed on it. Mixtures that are suitable for separation by chromatography include inks, dyes and coloring agents in textile material. As the paper absorbs the solvent, the different components of the dyestuff in the dyes react differently to it. These different spots of color will separate out, allowing you to see exactly what the components of the colors were. You can then use the spot to draw a picture to try to notice the different colored dyes that you identified in the chromatography. The colorant is a pigment or dye that gives its specific color. Whether black, blue, red, neon, yellow, pink or any other hue, the colorant is what you actually see when you look at a line made by a marker.
Chromatography may be preparative or analytical. The purpose of preparative chromatography is to separate the components of a mixture for later use, and is thus a form of purification. Analytical chromatography is done normally with smaller amounts of material and is for establishing the presence or measuring the relative proportions of analyses in a mixture. The two are not mutually exclusive. (02)
“Chromatography”; “chroma” from the Greek word for “color”, combined with “graphy”, meaning writing or recording. (03)
The word chromatography means “color writing” which is a way that a chemist can test liquid or solid mixtures. While studying the coloring materials in plant life, a Russian botanist invented chromatography in 1903. His name was M.S. Tswett.
History
The history of chromatography spans from the mid-19th century to the 21st. The term is chromatography, literally “color writing”.(03) Chromatography was first employed in Russia by the Italian-born scientist Mikhail Tswett in 1900. (04) He continued to work with chromatography in the first decade of the 20th century, primarily for the separation of plant pigment such as chlorophyll (which is green), Carotenes (which are orange), and Xanthophylls (yellow). Since these components have different colors (green, orange, and yellow, respectively) they gave the technique its name. New types of chromatography developed during the 1930s and 1940s made the technique useful for many separation processes.(05) Chromatography is such an important technique that two Nobel prizes have been awarded to chromatographers. Over 60% of chemical, dyestuff or pigments are analyzed worldwide thoroughly done with chromatography or a variation thereon.
Chromatography technique developed substantially as a result of the work of Archer John Porter Martin and Richards Laurence during the 1940s and 1950s, for which they won the 1952 Nobel Prize in chemistry. (06) They established the principles and basic techniques of partition chromatography, and their work encouraged the rapid development of several chromatographic methods Paper chromatology, Gas Chromatology, and what would become known as High Performance liquid chromatology. Since then, the technology has advanced rapidly. Researchers found that the main principles of Tswett’s chromatography could be applied in many different ways, resulting in the different varieties of chromatography described below. Advances are continually improving the technical performance of chromatography, allowing the separation of increasingly similar molecules.(06)
Chromatography is used by many different people in many different ways. Chromatography is based on differential migration. The solutes in a mobile phase go through a stationary phase. Solutes with a greater affinity for the mobile phase will spend more time in this phase than the solutes that prefer the stationary phase. As the solutes move through the stationary phase they separate. This is called chromatographic development
There are four two types of chromatography. These are –
A – Planar Chromatography (Stationery Phase)
B – Physical state of mobile Chromatography
How it works:
In all chromatography there is a mobile phase and a stationary phase. The stationary phase is the phase that doesn’t move and the mobile phase is the phase that does move. The mobile phase moves through the stationary phase picking up the compounds to be tested. As the mobile phase continues to travel through the stationary phase, it takes the compounds with it. At different points in the stationary phase the different components of the compound are going to be absorbed and are going to stop moving with the mobile phase. This is how the results of any chromatography are gotten, from the point at which the different components of the compound stop moving and separate from the other components.
In paper and thin-layer chromatography the mobile phase is the solvent. The stationary phase in paper chromatography is the strip or piece of paper that is placed in the solvent. In thin- layer chromatography the stationary phase is the thin-layer cell. Both these kinds of chromatography use capillary action to move the solvent through the stationary phase. What is the Retention Factor RF ? The retention factor, Rf, is a quantitative indication of how far a particular compound travels in a particular solvent. The Rf value is a good indicator
of whether an unknown compound and a known compound are similar, if not identical. If the Rf value for the unknown compound is close or the same as the Rf value for the known compound then the two compounds are most likely similar or identical.
In chromatography, the retardation factor (R) is the fraction of an analyze in the mobile phase of a chromatographic system. In planer chromatology in particular, the retardation factor Rf is defined as the ratio of the distance traveled by the center of a spot to the distance traveled by the solvent front. (07) Ideally, the values for RF are equivalent to the R values used in column chromatography (07)
The Retention factor, Rf, is defined as, Rf = distance the solute (D1) moves divided by the distance traveled by the solvent front (D2)
Rf = D1 / D2
Where, D1 = distance that color traveled, measured from center of the band of color to the point where the color was applied
D2 = total distance that solvent traveled
The Different Types of Chromatography
1. Liquid Chromatography
It is used in the world to test water samples to look for pollution in lakes and rivers. It is used to analyze metal ions and organic compounds in solutions. Liquid chromatography uses liquids which may incorporate hydrophilic, insoluble molecules. Liquid chromatography (LC) is a separation technique in which the mobile phase is a liquid. It can be carried out either in a column or a plane. Present day liquid chromatography that generally utilizes very small packing particles and a relatively high pressure is referred to as High performance Liquid Chromatography (HPLC).
2. Gas Chromatography
It is used in airports to detect bombs and is used is forensics in many different ways. It is used to analyze fibers on a person’s body and also analyze blood found at a crime scene. In gas chromatography helium is used to move a gaseous mixture through a column of absorbent material.
Gas chromatography (GC), also sometimes known as gas-liquid chromatography, (GLC), is a separation technique in which the mobile phase is a gas. Gas chromatographic separation is always carried out in a column, which is typically “packed” or “capillary”. Packed columns are the routine work horses of gas chromatography, being cheaper and easier to use and often giving dequate performance. Capillary columns generally give far superior resolution and although more expensive are becoming widely used, especially for complex mixtures. Both types of column are made from non-adsorbent and chemical inert materials. Stainless steel and glass are the usual materials for packed columns and quartz or fused silica for capillary columns
3. Thin-layer Chromatography
The first developments in thin layer chromatography occurred in the 1940s, and techniques advanced rapidly in the 1950s after the introduction of relatively large plates and relatively stable materials for sorbent layers.(08)
It uses an absorbent material on flat glass or plastic plates. This is a simple and rapid method to check the purity of an organic compound. It is used to detect pesticide or insecticide residues in food. Thin-layer chromatography is also used in forensics to analyze the dye composition of fibers.
Thin-layer chromatography (TLC) is a widely employed laboratory technique used to separate different biochemicals on the basis of their relative attractions to the stationary and mobile phases. It is similar to paper chromatography. However, instead of using a stationary phase of paper, it involves a stationary phase of a thin layer of adsorbent like silica gel, alumina, or cellulose on a flat, inert substrate. TLC is very versatile; multiple samples can be separated simultaneously on the same layer, making it very useful for screening applications such as testing drug levels and water purity and also using for dye compositions. (09)
Possibility of cross-contamination is low since each separation is performed on a new layer. Compared to paper, it has the advantage of faster runs, better separations, better quantitative analysis, and the choice between different adsorbents. For even better resolution and faster separation that utilizes less solvent, High-Performance TLC can be used. An older popular use had been to differentiate chromosomes by observing distance in gel (separation of was a separate step)
4. Paper Chromatography
It is one of the most common types of chromatography. It uses a strip of paper as the stationary phase. Capillary action is used to pull the solvents up through the paper and separate the solutes.
Affinity chromatography Affinity chromatography is based on selective non-covalent interaction between an analytic and specific molecule [09]. It is very specific, but not very robust. It is often used in biochemistry in the purification of proteins bound to tags. These fusion proteins are labeled with compounds such as His-tag, biotins or antigens, which bind to the stationary phase specifically. After purification, some of these tags are usually removed and the pure protein is obtained.
Affinity chromatography often utilizes a biomolecule’s affinity for a metal (Zn, Cu, Fe, etc.). Columns are often manually prepared. Traditional affinity columns are used as a preparative step to flush out unwanted bio-molecules. However, HPLC techniques exist that do utilize affinity chromatography properties. Immobilized Metal Affinity Chromatography (IMAC) is useful to separate aforementioned molecules based on the relative affinity for the metal (i.e. Dionex IMAC). Often these columns can be loaded with different metals to create a column with a targeted affinity.
Conclusion
Chromatography is an important biophysical technique that enables the separation, identification, and purification of the dyes components or pigments of a mixture for qualitative and quantitative analysis. Dyes or Dyestuffs can be purified based on characteristics such as size and shape of surface, and binding capacity with the stationary phase. Four separation techniques based on molecular characteristics and interaction type use mechanisms of ion exchange, surface adsorption, partition, and size exclusion. Other chromatography techniques are based on the stationary or mobile phase, thin layer and paper chromatography.
In addition, in this competition we justified the proper product compound and structure of the product. It should know us for the identification. Chromatography is used in many different ways. Some people use chromatography to find out what is in a solid or a liquid. It is also used to determine what unknown substances are. The Police, F.B.I., and other detectives use chromatography when trying to solve a crime. It is also used to determine the presence of cocaine in urine, alcohol in blood, PCB’s (polychlorinated biphenyls) in fish, and lead in water.
A wide range of chromatographic procedures makes use of differences in size, binding affinities, charge, and other properties. Many types of chromatography have been developed. These include Column chromatography, High performance liquid chromatography (HPLC), Gas chromatography, Size exclusion chromatography, Ion exchange chromatography etc
References
1. McMurry J (2011). Organic chemistry: with biological applications (2nd ed.). Belmont, CA: Brooks/Cole. pp. 395. ISBN 9780495391470.
2. Hostettmann K, Marston A, Hostettmann M (1998). Preparative Chromatography Techniques Applications in Natural Product Isolation (Second ed.). Berlin, Heidelberg: Springer Berlin Heidelberg. p. 50. ISBN 9783662036310.
3. “Chromatography”. Online Etymology Dictionary.
4. Ettre LS, Zlatkis A, eds. (26 August 2011). 75 Years of Chromatography: A Historical Dialogue. Elsevier. ISBN 978-0-08-085817-3.
5. Ettre LS, Sakodynskii KI (March 1993). “M. S. Tswett and the discovery of chromatography II: Completion of the development of chromatography (1903–1910)”. Chromatographia. 35 (5–6): 329–338. doi:10.1007/ BF02277520.
6. “The Nobel Prize in Chemistry 1952”. nobelprize. org. Retrieved 25 August 2016.
7. IUPAC, Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”) (1997). Online corrected version: (2006–) “Retardation factor, R in column chromatography”. doi:10.1351/goldbook.R05352
8. Touchstone, pp. 1651–1652
9. Wilchek M, Chaiken I (2000). “An overview of affinity chromatography”. In Bailon P, Ehrlich GK, Fung WJ, Berthold W (eds.). Affinity
Chromatography. Methods in Molecular Biology. 147. Humana Press. pp.