Abstract:-
During the last few years, easy care concept has expanded rapidly to include Oil and water resistant features in many apparel applications. Easy care concept now encompasses both minimizations of ironing and easiness to clean garments that get stained in day to day use. Fabric manufacturers and chemical manufacturers have made significant advances in finishing processes to ease stain removal and prevent stain penetration Substrates that possess water and oil repellency are desirable for in many textile applications. Water repellency is increasingly becoming the focus of interest for protective clothing. This repellency can be achieved by implementing water repellent chemicals on textile fibres with minimal effects on other functional properties like strength, flexibility etc. Water and oil repellant fabrics can be used for a variety of end uses such as outerwear, where the requirement is for a high degree of water repellency and general wear such as expensive silk sarees, where the focus is more both water and oil repellency. Other common end uses for these finishes include upholstery, rugs, carpets, protective clothing Filter fleece, Uniforms, Table cloths, wallpaper, etc, etc.
Introduction
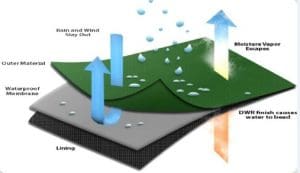
Water repellency is defined as the ability of a textile material to resist wetting. In principle, water repellent treatments are based on the deposition of hydrophobic substances on the fibre. The difference between waterproof and water repellent finishes is that in the former the interstices between warp and weft yarns are completely blocked by the continuous hydrophobic film of the substance and not only water pass through the fabric, but the fabric will become impermeable to air also. Water repellent finish, on the other hand, is one in which the material is made water repellent without filling up the interstices of fabric completed which results in fabric permeable to air but not to water. Mechanical treatment, chemical treatment and coating methods are three main methods for imparting water repellency to textiles. In these processes, generally durable repellency effects are obtained. In addition to the desired repellency effects, other undesirable fabric properties are often found with repellent finishes. These include problems with static electricity, stiffer fabric hand, greying during aqueous laundering and increased flammability. Research is going on to reduce the above-mentioned problems and obtain a durable water repellent fabric.

Quality of Water Repellent Finish
- The product should impart water repellent finish for the majority of fibre types although there is not available any universal agent for all fibres.
- The finished fabric should be durable to washing and resistant to dry cleaning
- Easy handling and storing
- Low foaming
- High resistance to yellowing tendency making it suitable for both white and colored goods.
- It should not affect adversely the light fastness of shade
- The fabric finish should not become very stiff and harsh.
Mechanism
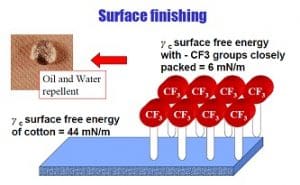
Repellent finishes achieve their properties by reducing the free energy at fibre surfaces. If the adhesive interactions between a fibre and a drop of liquid placed on the fibre are greater than the internal cohesive interactions within the liquid, the drop will spread. If the adhesive interactions between the fibre and the liquid are less than the internal cohesive interactions within the liquid, the drop will not spread. Surfaces that exhibit low interactions with liquids are referred to as low energy surfaces. Their critical surface energy or surface tension γC must be lower than the surface tension of the liquid γL (the internal cohesive interaction) that is repelled.
For fabrics to be water repellent, the Surface free energy of the fibre’s surface must be lowered to about 24 to 30 mN/m. Pure water has a surface tension of 72 mN/m so these values are sufficient for water repellency.
Oil repellency requires that the fibre surface be lowered to 13 mN/m. Only fluorochemicals are able to function as oil repellents so whatever is mixed with them must not interfere with how they are deposited. Therefore, oil repellency finishes with fluorocarbons (yC = 10-20 mNm-1) always achieve water repellency but fluorine free products, for example silicones (yC=24-30mNm-1) will not repel oil.
| Solid | Surface Free energy mN/m |
| Polyamide (Nylon 6.6 ) | 46 |
| Cotton | 44 |
| Polyester | 43 |
| PVC | 39 |
| Polyethylene | 31 |
| Polysiloxane, typical silicone oil | 23-24 |
| PTFE | 18 |
Fluorochemical repellents are unique in that they confer both oil and water repellency to fabrics. The ability of fluorochemicals to repel oils is related to their low surface energy which depends on the structure of the fluorocarbon segment, the nonfluorinated segment of the molecule, the orientation of the fluorocarbon tail and the distribution and amount of fluorocarbon moiety on fibres. Low surface energy can be described in critical surface tension terms.
Approaches to impart Water Repellency to Fabrics
- Spray-on and wash-in products:There are the number of temporary solutions for creating waterproof (or ‘spill-proof’ to be precise) solutions for garments and fabrics by sprays or additives in home laundries. These solutions, although effective for a one-off use, are not for long term and tend to diminish in effect in a few washing cycles.
- Fabric finishes:Finishes are the most common and widely used approach for imparting durable water repellency on fabrics and garments. These finishes (commonly called DWR finishes) are performed after the fabric is constructed. The oldest water repellent finishes for fabrics started with coatings of paraffin or wax but they used to wash out eventually. Recent finishes mainly involved fluorocarbon based chemistries. Perfluorocarbons (PFC’s) are capable of repelling water, oil and other liquids that cause stains. However, their toxic effects and bioaccumulation have been a major ecological concern.
- Yarn based solutions:Yarn based solutions have not deviated much from fabric finishes, in terms of chemistry, but they are focused more on treating the yarn instead of the fabric. This approach not only provides better protection from stains but also help in maintaining the breathability of the fabric similar to without the treatment.
- Water proof membranes:Water proof membranes are typically made of PTFE (poly tetra-fluoro ethylene) and related compounds. These are the same fluorinated compounds found in non-stick cookware and paints and coatings. The outdoor market utilizes a range of such solutions which resist water to penetrate the fabric but maintain the breathability to an acceptable level.
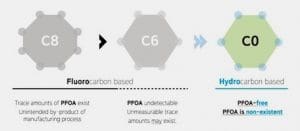
- Fluorocarbon based Water RepellentFluorinated water repellents are widely used on textile products due to their outstanding ability to protect against water, oil and soil. Fabrics treated with fluorinated water repellents are suitable for outdoor clothing to help provide excellent durability.Fluorocarbons (FC) provide fibre surfaces with the lowest surface energies of all the repellent finishes in use. Both oil and water repellency can be achieved. FC repellents are synthesised by incorporating perfluoro alkyl groups into acrylic or urethane monomers that can then be polymerised to form fabric finishes. Originally, the perfluoro alkyl groups were produced by electrochemical fluorination, but today they are produced by telomerisation. The telomerization process produces primarily or exclusively linear PFASs, whereas the electrochemical fluorination process produces a mixture of branched and linear isomers. The final polymer, when applied to a fibre, should form a structure that presents a dense CF3 outer surface for maximum repellency. General advantages of fluorocarbon-repellent finishes include low active addons (< 1 % owf) and more rapid drying of treated fabrics. Special FCs allow improved soil release during household laundering or stain resistance on nylon, which is especially useful for carpets.They also have excellent chemical and thermal stability which provides treated fabrics with good durability (e.g., during laundering and dry-cleaning. Most repellents based on this chemistry are applied by padding process and then dried and cured.The presence of fluorine, the most electronegative atom, allows PFC-based DWRs and other fluorosurfactants to reduce the surface tension of the fabric to lower than that of water and oil. The unique ability to repel oils as well as water has been a major contributor to the popularity of PFC-based finishes.C8 Chemistry – The backbone of these compounds is made of a chain of 8 carbon atoms. Two methods are used to produce two slightly different products, namely “electro-fluorination” (electrolysis to replace hydrogen atoms in a molecule by fluorine atoms to create the 8 unit chain containing just carbon and fluorine) and “telomerisation” (joining single units together on to the growing polymer chain). The molecules, PFOA (perfluoro octanoic acid) and PFOS (perfluoro octanoic sulphate), so produced, have been found accumulated in various animals and have adverse effects, including carcinogenicity and toxicity. As a result of their strong carbon-fluorine bonds, PFOA and PFOS do not break down in the environment. They have been shown to be persistent in the environment and have long elimination half-life in wildlife and in humans.
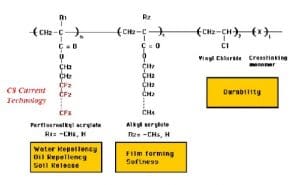
The good news is there’s a way to limit the negative side effects of fluorocarbons to a minimum. The solution lies in reducing the fluorocarbon chain from C8 to C6.
C6 Chemistry – C6 based fluorocarbons were introduced to minimize the release of toxic chemicals. C6 based fluorocarbons showed decreased water repellency than C8 based fluorocarbons ones. C6 Chemistry – PFHA (perfluorohexanoic acid) with backbone of 6 carbon atoms, is supposed to be 40 times less bio-accumulative than PFOA (the 8-carbon counterpart). But it is also less effective, so more of the chemical has to be used to achieve the same result. Additionally, the recipe also involved small traces of C8 molecules. At present C6 technology is most prevalent in the textile industry although a growing number of sustainability-conscious brands are phasing out the use of such chemicals in their products.
Summary of hazards associated with C8 against C6 based water repellent
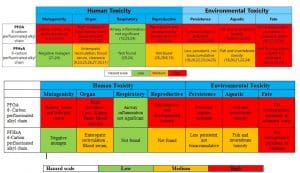
Mode of action C6 vs C8
This alternative doesn’t have the exact same properties as the original fluorocarbons, but the side products are way less persistent and bio-accumulative, meaner they’re less harmful. Many chemical companies, including ourselves, have stopped working with C8 altogether and started working with C6 fluorocarbons exclusively. Moreover, governmental organizations all around the world have strongly commended manufacturers to stop using C8, meaning that in the long run, the chemistry world must look for alternatives anyway.
Types of C6 based water repellent compounds
| Product | WR | OR | Softness |
| Current C8 Commercial Product | Excellent | Excellent | Good |
| C6 Hybrid | Excellent | Excellent | Good |
| C6 Non Hybrid | Excellent | Excellent | Poor |
| C6 Non Hybrid Silicone Blend | Excellent | Poor | Fair |
| C6 Copolymer with silicone | Excellent | Poor | Fair |
Smaller chemistries: Attempts to use smaller perfluorocarbon segments (e.g. C4) have been made by many manufacturers. The smaller the fluorocarbon, the more rapidly it breaks down in the environment (a positive trait) but, unfortunately, the desired textile performance goes down as the size of the perfluorocarbon goes down.
Greener Approaches C0
The adverse effects of DWR chemistries involving fluoropolymers are well known in the textile industry and steps are being taken towards more sustainable and greener approaches.
Types of C0 based Water Repellent
Over the last century, many chemical finishes have been introduced which can impart water repellency to textile fabrics. Product formulations have included metal-salt finishes, soap/metal salt-finishes, wax finishes, pyridinium-based finishes, organo-metallic complexes, fat-modified melamine resins, silicones and fluorocarbons finishes. Over the last 20 years the focus in chemical finishing has shifted towards multifunctional finishes which not only confer durable water repellency but also oil-, soil- and stain-repellency. These achieved the high standards of performance demanded in many branches of the textile and apparel industries, for example, outdoor performance clothing, military textiles and many types of technical textiles.
- Parrafin Repellents
These were one of the earliest water repellents used, but they do not repel oil. Typically the products are emulsions that contain aluminium or zirconium salts of fatty acids. The paraffinic portion of the repellent mixture is attracted to the hydrophobic regions, while the polar ends of the fatty acid are attracted to the metal salts at the fibre surface. These finishes can be applied by both exhaustion and padding. They are compatible with most kinds of finishes but they increase flammability.
Although they are available at relatively low cost and generate uniform waterproof effects, the lack of durability to laundering and dry cleaning and their low air and vapour permeability limits the use of paraffin-based repellents.
- Stearic Acid-Melamine Repellents
They are compounds formed by reacting stearic acid and formaldehyde with melamine and constitute another class of water-repellent materials. The hydrophobic character of the stearic acid groups provides the water repellency, while the remaining N-methylol groups can react with cellulose or with each other (crosslinking) to generate permanent effects. Advantages of the stearic acid–melamine repellents include increased durability to laundering and a full hand imparted to treated fabrics. Disadvantages of stearic acid–melamine repellents include problems similar to durable press finishes (a tendency to exhibit finish mark-off, decreased fabric tear strength and abrasion resistance, changes in the shade of dyed fabric, and release of formaldehyde).
- Silicone Water Repellents
Polydimethylsiloxane products are useful as water repellents and form a hydrophobic layer around fibres. In order to gain some measure of durability, silicones designed as water-repellent treatments usually consist of three components, a silanol, a silane and a catalyst such as tin octoate. The outward oriented methyl groups generate the water repellency. During the drying step after pad application, the silanol and silane components can react to form a three-dimensional crosslinked sheath around the fibre. The Si–H groups of the silane are the reactive links in the silicone chain, generating crosslinks or being oxidised by air or hydrolysed by water to hydroxyl groups. Advantages of silicone water repellents include a high degree of water repellency at relatively low ( 0.5–1 % owf) on weight of fabric concentrations, very soft fabric hand, improved sewability and shape retention, and improved appearance and feel of pile fabrics.
The disadvantages of silicone repellents include increased pilling and seam slippage, reduced repellency if excessive amounts are applied, only moderate durability to laundering and dry cleaning, and no oil and soil repellency. The silicone finish may enhance the attraction of hydrophobic dirt. In addition, the waste water, especially the residual baths, from these finish application processes are toxic to fish.
Fluorine-free Chemistries: Although products with no PFOA and PFOS claim to be fluorine-free DWR products, there are products available or being tried that involve completely different chemistries. Paraffin (and other hydrocarbon based solutions), silica nanoparticles, Silanes (e.g. alkyl trialkoxy silanes) and are some of the front running examples.
Dedrimer
Dedrimer based repellent chemistry is a relatively new field of repellent chemistry. Dedrimers are characterized by regular hyperbranched monomers leading to monodisperse tree like structures. Historically dedrimers have been used in the field of genetics, medicines, biology, and chemistry. In textile chemistry finishes containing dedrimers are applied to fabrics to impart water and oil repellency properties.
Nanotechnology
Repellent chemistries containing nano-materials are coated on fabrics to achieve desirable properties without a significant increase in weight, thickness or stiffness. The proerties that can be imparted on textiles using nanotechnology include water repellency and soil resistance. The use of chemistries containing nano materials to impart water repellency and stain sersistance effects on textiles is one of the most common ways nanotechnology is being used in textile industries.
Plasma Technology
Plasma treatment attracts particular interest especially for its main peculiarities: the treatments interest only the uppermost layers of the fabric surface without modifying the bulk properties and it is environmental friendly, since the use of chemicals is negligible. The utilization of low-pressure plasma processes, in particular, has been widely investigated for the modification of surface properties of textile composed by synthetic polymers and natural materials. Plasma processing can also impart a repellent finish on textile and does not require high levels of thermal energy because there is no water to evaporate and the fluoropolymer polymerizes in the plasma therefore it does not need to be cured. Until recently, plasmas for industrial processing were only available under reduced pressure. This limited manufacturing because of the high cost of the vacuum equipment and the limitations of batch processing.

Evaluation of Water Repellent Textiles
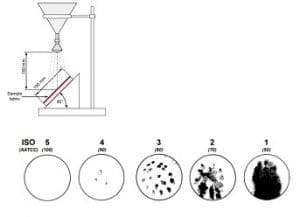
AATCC 22 – Water repellency: spray test
Procedure
Treated fabric is stretched taut, held at a 45º angle and sprayed with 250 ml of water from above. The resulting wetting pattern, if any, is rated using photographic standards. This is a simple, rapid method suitable for the plant floor
Other Test Methods
| TEST METHOD | PROCEDURE SUMMARY |
| AATCC TM 42 – Water resistance: impact penetration test | Similar to AATCC TM 22, but a weighed piece of blotter paper is placed under the fabric. The weight gain of the paper after 500 ml of water has been sprayed on the fabric is recorded. |
| AATCC TM 35 – Water
resistance: rain test |
The treated fabric, backed by a weighed piece of blotter paper, is sprayed with water under constant hydrostatic pressure for 5 min. The weight gain of the paper after the test is recorded. This test requires a special apparatus. |
| ISO 9865 (DIN 53 888) Bundesmann rain-shower test | Four samples of treated fabric are subjected to simulated rain for 10 min. The fabrics (placed on inclined cups and sealed at the edges) are in constant motion and the side of the fabric not exposed to the rain is subjected to a rubbing action. The repellency of the fabric is determined by the appearance of the wetted side, the amount of water absorbed by the fabric and the amount of water passing through the fabric. This test requires an elaborate special apparatus. |
| AATCC TM 127 and ISO 811 or EN 20 811 – Water resistance: hydrostatic pressure test (former DIN 53886, Schopper- Schmerber test) | One surface of the treated fabric is subjected to a constantly increasing hydrostatic pressure until three points of leakage appear on the opposite surface. The pressure at the third point of leakage or is recorded in centimetres or metres on a water gauge. A static pressure variation of this test determines the time until the named leakage occurs at a given pressure. This test requires a special apparatus. |
| DuPont water repellency test | Similar to AATCC TM 118, but with water-propan2-ol mixtures with increasing alcohol content. |
| AATCC TM 22-water repellency: spray test | Water sprayed against the taut surface of a treated fabric under controlled conditions produces a wetted pattern. The size of the wetted pattern which depends on the relative repellency of the fabric is compared to a standard chart of fabric water repellency ratings of zero (0), 50, 70, 80, 90 and 100. A rating of zero (0) is assigned if the fabric’s surface is completely wetted by water, whereas a rating of 100 corresponds to no wetting of water on the surface of the fabric |
| EN 14360–rain test (test method for ready-made garments) | This test method is a European standard that defines test conditions under which ready-made garments are exposed to heavy rain. It applies to garments such as jackets, trousers, coats, etc. This test method does not apply to the testing of garments for resistance to other weather conditions such as snow or strong winds. |
| AATCC TM 193-aqueous liquid repellency: water/alcohol solution resistance test | Drops of a selected series of water/alcohol solutions of different surface tensions are placed on a treated fabric surface and observed for wetting. This test method is used to evaluate the effectiveness of the finish in imparting a low surface energy on the surface of the treated fabric. |
| AATCC TM 118-oil repellency: hydrocarbon resistance test | Drops of eight selected liquid hydrocarbons of different surface tensions are placed on a treated fabric and observed for wetting. The oil repellency grade of the fabric is the highest numbered test liquid which does not wet the fabric surface with the highest achievable grade being 8. This test method is used to detect the presence of a finish capable of imparting a low energy surface on the treated fabric |
| AATCC TM 130-soil release: oily stain release method | A stain applied on the treated fabric is forced into the fabric using a specified weight. The stained fabric is then laundered in a prescribed manner and the residual stain is compared to a graduated series of stains. This test method measures the ability of the fabric to release oily stains during home laundering. |
Conclusion
Mankind in the twenty first century has begun to feel a grave need to use resources in a sustainable manner; environment depletion and deterioration has increased manifold due to extreme materialism. Fast production, consumption and discarding of products are leading to an outbreak of problem of heaping toxic landfills. People are seen shifting towards ecofriendly products because of the harm that they have continued to do to their environment for fulfillment of their needs.
In the current market, technical fabrics by well-known brands, including protective textiles, are coated using perfluorocarbons (PFCs), which give them highly durable oil-repellent and water-repellent properties. However, these products are harmful to health and the environment. Consequently, these chemicals based on PFOS and PFOAS – known as C8 chemicals – are under pressure from legislation. The aim of this legislation is to replace them with C6 or C4 chemicals or even with Czero, however these products provide lower performance in terms of durability and resistance. Despite intensive research, no alternative to PFC has yet been found for successfully making apparel oil-repellent. This is why there is a great demand for equivalent high-performance alternatives to C8 products.
Authored by-
Mr. Edward Menezes (Chairman)
Rossari Biotech Ltd

