Study on Physio-chemical analysis of Textile industry Effluent and in vitro study of decolourization of the Congo Red Acidic dye by bacterial species
- P. SONTAKKE*, A. K MISHRA** AND K.S. Muralidhara***
*Jr Quality Assurance Officer, Textiles Committee, Min of Textiles, Govt. of India
**Lecturer, Agnihotri College Of Science and Biotech Research Center, Wardha
*** Joint Director (Laboratories), Laboratories, Textiles Committee, Min of Textiles, Govt. of India, Mumbai, India
Abstract: Decolourization study was carried out by treating the textile dye effluent with the bacteria strains namely Klebsiella sp Staphylococcus sp, Psedomonas sp and Bacillus sp. isolated from highly polluted drain, locally known as Buddha Nala, Ludhiana. The pollution load of the various textiles industrial effluents vary from time to time depends upon the dyes, impurities on the fabrics and other processing chemicals used for the dyeing over the years, with the active spread and development of the industries. Heavy Metal, which are either used, or produced as by products by numerous manufacturing, industrial, refining and mining processes have become ubiquitous, persistent environmental pollutants. This study to isolate and optimize bacterial strains having the ability to degrade and decolorize azo dyes produced in the final effluent of textile dying industries and also the physiochemical pollution load at textile dying industries. It has a great disadvantage in terms of its environmental impact because it consumes considerably high amount of processed water and produces highly polluted discharge water. Joints efforts are needed by water technologists and textile industry experts to reduce water consumption in the industry. While the user industries should try to optimize water consumption, water Technologists should adopt an integrated approach to treat and recycle water in the industry in the name of environmental protection.
Keywords: Textile industry effluent, Microbial biotechnology, Technical Textiles, Azo dye decolourization, bacteria
- INTRODUCTION:
Water being one of the key inputs, textile industry consumes large quantity of quality water. Since about 90% of the chemicals used in textile processing are eliminated after completing goals. The effluent carries with it a high polluting load. Textile industries are large industrial consumers of water as well as producers of wastewater. To produce 1 ton of textile product industry consume 200-270 tonnes of water. Increased demand for textile products, lead to increase in the generation of textile effluent, which makes the textile industry as a main sources of severe pollution problems worldwide. The process of dyeing involves the use of different chemicals like salts, metals, surfactants, sulphide and formaldehyde. There are more than 8,000 chemical products associated with the dyeing process and over 100,000 commercially available dyes exist with over 7×105 metric tons of dyestuff produced annually [1]. Nearly, 1,000-3,000 m3 of water is let out after processing about 12-20 tonnes of textiles per day. These effluents are rich in dyes and chemicals, many of which are non-biodegradable and carcinogenic and pose a major threat to health and the environment [2]. Wastewater generated in different production steps of a textile mill have high pH, temperature, detergents, oil, suspended and dissolved solids, dispersants, levelling agents, toxic and non-biodegradable matter, color and alkalinity. Important pollutants in textile effluent are mainly recalcitrant organics, color, toxicants and surfactants, chlorinated compounds.
Textile effluent includes a large variety of dyes and chemicals additions that create ecological challenge not only as liquid waste but also as chemical composite. Main pollutants in textile effluent come from dyeing and finishing processes. These processes require the input of a wide range of chemicals and dyestuffs, which generally are organic compounds of complex structure. Dyes contributed to overall toxicity at all process stages. Also dye baths could have high level of BOD/COD, colour, toxicity, surfactants, fibers and turbidity and may contain heavy metals [3]. Major pollutants in textile wastewaters are high suspended solids, COD, heat, colour, acidity, and other soluble substances [4].
Color in the effluent is one of the most obvious indicators of water pollution. The discharge of highly colored synthetic dye effluents is aesthetically displeasing and can damage the receiving water body by impeding penetration of light. Moreover, highly coloured wastewaters can block the penetration of sunlight and oxygen, essential for the survival of various aquatic forms.Dyes are recalcitrant molecules which are difficult to degrade biologically. Azo dyes are designed to resist chemical and microbial attacks and to be stable to light and during washing. Some of azo dyes are either toxic or mutagenic and carcinogenic in nature. The diversity in composition of chemical reagents used in textile industries contributes to severe water pollution. In some cases, the dye solution can also undergo anaerobic degradation to form potentially carcinogenic compounds that can end up in the human food chain. Complete characterization of effluents is essential to identify the suitable treatment method and in turn support the better design of treatment plant.
To overcome the difficulties posed by conventional wastewater treatment systems bioremediation has emerged as a promising technology in the past few years for the treatment of industrial dye effluents and contaminated soil. Biotechnology harnesses the catalytic power of biological systems, whether by direct use of enzymes or through the use of the intricate biochemistry of whole cells and micro-organisms. The ability of microbes to degrade a vast array of pollutants makes bioremediation a technology that can applied in different soil conditions. Some of examples bioremediation technologies are mycoremediation, phytoremediation, bioventing, bioleaching, landframing, bioreactor, composting, bioaugmentation, rhizofiltration, and biostimulation. Dyes can be degraded and decolorized by various microorganisms such as bacteria, fungi, yeast etc. Bacteria could also degrade synthetic dyes at a faster rate but at the same time releases carcinogenic aromatic amines as degradation products which severely affects human and other animal health. A new type of bioremediation (the successful transformation of toxic polluting agents to non-toxic, usable substrates for further ‘macro’-degradation) has become a major research now a day.
Out of various activities in textile industry, chemical processing contributes about 70% of pollution. Due to the nature of various chemical processing of textiles, large volumes of waste water with numerous pollutants are discharged. Such pollution is particularly associated with the reactive azo dyes and our aim is to adopt technologies giving minimum environmental pollution. As a result, Ludhiana is one of the most industrialized cities in India, was selected for present study. It is known as the textile capital of North India and associated with the wastewater from several textile mills in the woven fabric and knit fabric finishing industry and one highly polluted drain, locally known as Buddha Nala, According the Punjab State Department of Fisheries the pollution of the Buddha Stream has led to the drastic reduction in the fish yield in river Satluj. Now it has no fish because of the high level toxicity in the water. So attempt is made initially to reduce the pollution of the Buddha Stream at in vitro level. In present approach, including physicochemical analysis of textiles effluent and microbes or their enzymes are being used to degrade toxic wastes instead of traditional processes, thus waste treatment is useful industrial asset of biotechnology.
In this study, Bacterial Strain was examined for its ability to decolourize the textile dye in industry wastewater. The basic step in the decolorization and degradation of azo dyes is breakdown of azo bonds, leading to removal of color. Azo dyes are known to undergo reductive cleavage whereas the resultant aromatic amines are metabolized under aerobic conditions. In view of these problems the most potent bacterial culture was selected in this study for maximum decolorization of Congo Red Acid dye, being selected as model azo dye.
- Materials and methods
- Sampling and Analysis of Effluent
- Sampling:
Highly polluted drain, locally known as Buddha Nala, Ludhiana was chosen for effluent sample collection. Standard procedures (Spot and Grab) were followed during sampling. Sampling site covers the effluent from 6 textile mills in the woven fabric and knit fabric finishing industry mostly using vegetable fibres such as cotton, animal fibres such as wool, silk, and synthetic materials such as nylon, polyester, and acrylics.
- Chemicals:
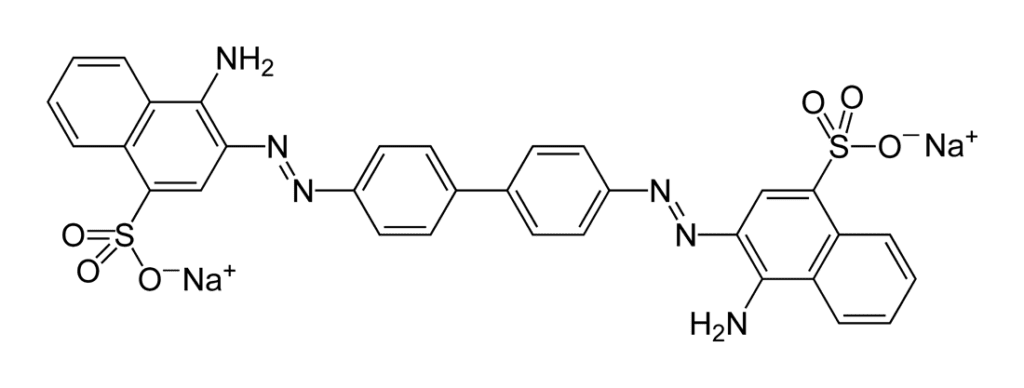
All media components and chemicals used in the present study were of analytical grade and purchased from Hi Media Laboratories (Mumbai, India).The dye Congo red an anionic azo dye IUPAC as 1-napthalenesulfonic acid, 3, 3-(4, 4- biphenylenebis (azo)) bis (4-aminodisodium) was obtained from local supplier and its stock solution was prepared in double-distilled water. All the test solutions were prepared by diluting the stock with double- distilled water.
Figure 1: Structure of Congo red dye
- Analysis of effluent: [5].
- Determination of Biochemical Oxygen Demand (BOD) [6].
- Determination of Chemical Oxygen Demand (COD) [6].
- Isolation and Identification of bacterial strains:
Isolation and identification of bacteria were carried out by plate counting technique. One gram of soil and sludge were weighed individually and suspended in 99ml of sterile distilled water. One ml was pipetted and serially diluted with 10 ml distilled water up to 10-6 dilutions. From this 0.1ml of was taken and spread onto nutrient Agar medium containing the following chemicals: Beef extract 3gmL-1, NaCl 5gmL-1, Peptone 5gmL-1 Agar 20gmL-1 and incubated at 37°C for 24hrs. Discrete bacterial colonies that developed on Agar plates were initially grouped on the basis of colony morphology, pigmentation followed by Gram staining and motility.
- Screening for Dye Decolorizers:
From 25 morphologically distinct strains isolated from the textiles effluent, only 4 isolates were found to possess the ability to decolorize the Dye Congo Red and marked them as ISOB1, ISOB2, ISOB3 and ISOB4. Dye degrading isolates were identified on the basis of morphological and biochemical tests according to Bergey’s Manual of Systematic Bacteriology Selected bacterial isolates were further purified and sub cultured for further study.
- Determination of bacterial growth:
The maximum growth rate of four bacterial isolates and consortium were measured spectro photometrically. Five loops of 24 hrs old culture was inoculated in to conical flask containing 100 ml sterile nutrient broth and incubated at 37°C. The bacterial growth curve was measured by turbidometric method, reading at 660 nm in regular time intervals.
- Screening of bacteria for azo dye decolourization:
Five loops of 12 hrs old bacterial pure isolates namely ISOB1, ISOB2, ISOB3 and ISOB4 were used as inoculants to degrade the Congo red. Conical flask assay was performed for the detection of decolorizing activity of bacteria. The nutrient broth was autoclaved at 121 0C for 15 minutes. 5 % inoculums of the selected culture showing maximum decolorizing activity was added to nutrient broth flasks, amended with 100 mg/l of Congo red dye containing. The flasks were covered with Aluminum foils and were incubated at 37 0C for 4 days.
- Biodecolorization and biodegradation analysis
The flasks were observed for decolorization of the azo dye present in the medium. At every 12hrs interval 5 ml aliquot of the decolorized culture broth was collected and centrifuged at 10,000 rpm for 5 minutes. The supernatant was recovered and analyzed spectrophotometrically at 497 nm.
The analysis was done using UV-VIS spectrometry, Decolourization extent was calculated using the following equation [7]
Where Co refers to the initial absorbance, Ct refers to the absorbance after incubation; and t refers to the incubation time.
- Effect of pH and temperature:
Decolorization was studied at varying pH (3.0–13.0) and temperature (20°C-50°C). The pH of the medium was adjusted using 0.1 N HCl or 0.1 N NaOH. Experiments were performed in 30 ml screw capped tubes containing 30 ml of above mentioned inoculated medium using only Potential isolate(ISOB4) under static culture condition.
- RESULTS AND DISCUSSION:
3.1 Physico-chemical analysis of textile effluent:
In the present investigation the colour of the effluent was Blackish brown to brown. In the present investigation pH values of effluents sample was 6.3. Rao et.al., also observed the pH of textile industry effluent varied from 5.0 to 11.0 [8]. pH value of the effluent was found to be significantly vary depends upon the dyes (acidic, basic and reactive dyes) and the substrate. The TSS values of effluent were found to vary significantly with Industries as well as with sampling days. In the present investigation TSS found to be 765 mg/l. The increased amount of TSS is due to increased chemical dosing dye fixation and partial dissolution of fibre materials. In other studies the amount of TSS in different textile wastewater samples was found to be in the range of 1020-3680 mg/l which is considerably higher than the result of our findings [9]. The total dissolved solids in sugar mill effluent, tannery waste and textile industries were also reported in the level of 400 – 1650 mg/l[10], 1000 – 2850 mg/l and 8500 – 15000 mg/l respectively.
Divalent metallic cations particularly Ca+2, Mg+2, Sr+2, and Fe+2 are responsible for hardness in textile effluents. Hardness of the effluent sample found to be 709 mg /l. Chloride in wastewater comes mostly from raw water taken for dyeing and also it may add as fixing agents for some of the dyes. Chloride also contributes to the increase in TDS. As reported by Agarwal[11] the industrial wastewater containing sulfate ions should not be discharged into any water body from where water is supplied for drinking, as higher concentration of sulfate ions cause taste change in water, have a laxative effect on livestock and humans, and are usually associated with high hardness levels.
Bicarbonates are directly related to total alkalinity i.e increase in carbonates and bicarbonates increases the total alkalinity. High pH values indicate alkalinity (bicarbonates) problem with sodium ion likely to be the dominant cation. Alkalinity of the effluent sample was found to be 1287 mg/l. Higher alkalinity is due to use of chemicals like Na2CO3, NaHCO3 and NaOH, surfactants and NaH2PO4. Textile industries use organic substances as raw materials and high levels of dissolved organic matter consume large amounts of oxygen and increase BOD level, which undergoes anaerobic fermentation processes leading to formation of ammonia and organic acids. In the present investigation BOD of textile effluents is 269 mg/l which is higher than the permissible limit (30 mg/L) of CPCB [6]. Increase in BOD which may cause hypoxia conditions with consequent adverse effects on aquatic biota. The COD levels obtained from the industries shows that detergents, softeners and impurities on the fabrics contributes a significant portion of the COD. Highest COD levels were obtained on dyeing indicating that in addition to fabric impurities removed during scouring or desizing and the contribution of detergents and softeners, residual dyes contributed a large proportion of the COD. COD of untreated textile effluents were 2389 mg/l and this value of the COD is beyond the permissible limit (250mg/L) of CPCB [6]. This indicates that the effluents were unsuitable for the existence of aquatic organisms due to the reduction of DO content.
3.2 Isolation and identification of bacterial strains:
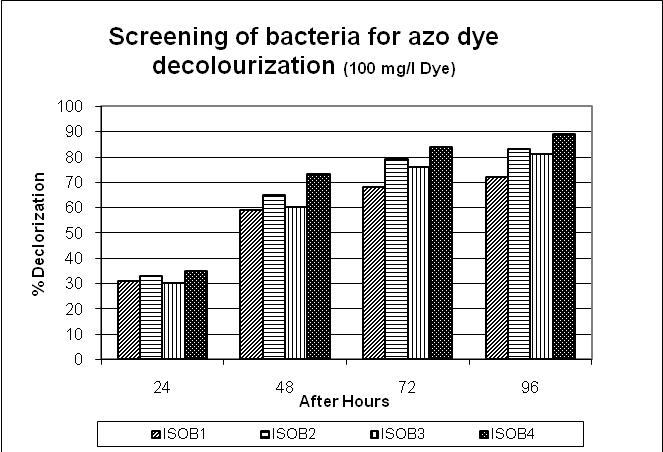
In the present study 5 morphologically distinct strains isolated from the textiles effluent and 4 isolates were selected for decolourization study based on their higher potential to decolorize the dye Congo Red and marked them as ISOB1, ISOB2, ISOB3 and ISOB4. The percentage decolourization found to be 68, 79, 76 and 84 respectively for isolate ISOB1, ISOB2, ISOB3 and ISOB4 after 72 hours of Incubation using concentration of 100 mg/l of Congo Red dye. And when used 200 mg/l of Dye the percentage decolourization found to be 67, 79, 76 and 82 respectively for isolate ISOB1, ISOB2, ISOB3 and ISOB4 after 72 hours of Incubation and 65, 77, 76 and 84 percentage of decolourization at 300 mg/l of Congo red Dye. [Fig. 2, 3, 4] The decolourizing activity of the bacterial consortium was studied using Congo Red Dye at different initial concentrations varying from 100 to 300 mg/l. The maximum decolourization was observed up to 200 mg/l. The maximum decolourization observed by isolate ISOB4 i.e.82 % followed by isolates ISOB2 (79%) ISOB3 (76%) and ISOB1 (67%)
Figure 2: Screening of bacteria for azo dye decolourization (100 mg/l Dye)
Figure 3: Screening of bacteria for azo dye decolourization (200 mg/l Dye)
Pure bacterial isolates ISOB1, ISOB2,ISOB3 and ISOB4 were identified as Klebsiella sp., Staphylococcus sp., Psedomonas sp and Bacillus sp. by respectively biochemical characteristics [Table:1] using standard microbiological procedures based on the methods of in Bergey’s Manual of Systematic Bacteriology. Daneshvar a et.al, reported, many bacteria capable of reducing Azo dyes which were isolated from textile effluent contaminated sites [10].
Wong & Yuen reported 100% dye removal of Methyl Red (initial conc., 100 mg/l) in 24 h by Klebsiella pneumoniae RS-13. Hu[10] also reported 93.2% dye removal of RBB (initial conc., 100 mg/l) in 48 h by Pseudomonas luteola. While comparing experimental isolates with these reports, it seems CPE and CPS did not perform sufficiently well but CBE and FBE are almost at par. Performance of FBE seems to be better than Pleurotus ostreatus sp.4, which causes 53% decolourization in 18 days with Poly R-478 dye (initial conc., 100 mg/l). Since CBE and FBE performed better than other two isolates, further experiments on decolourization of various dyes were performed with these strains. The reason for the decreased decolorization under shaking conditions could be competition of oxygen and dye compounds for the reduced electron carriers under aerobic conditions. The percentage decolorization of Crystal violet by Bacillus subtilis strain under static conditions was 90% within 24h of incubation which was equal to a similar study but with 35h of incubation period. In another study conducted with Pseudomonas putida. P. fluorescence, Bacillus cereus and Stentrophomonas acidaminiphila to decolorize Acid Red 88 showed their efficiencies at 35%, 31 %, 40% and 50% respectively [10].
3.3 Effect of pH and temperature:
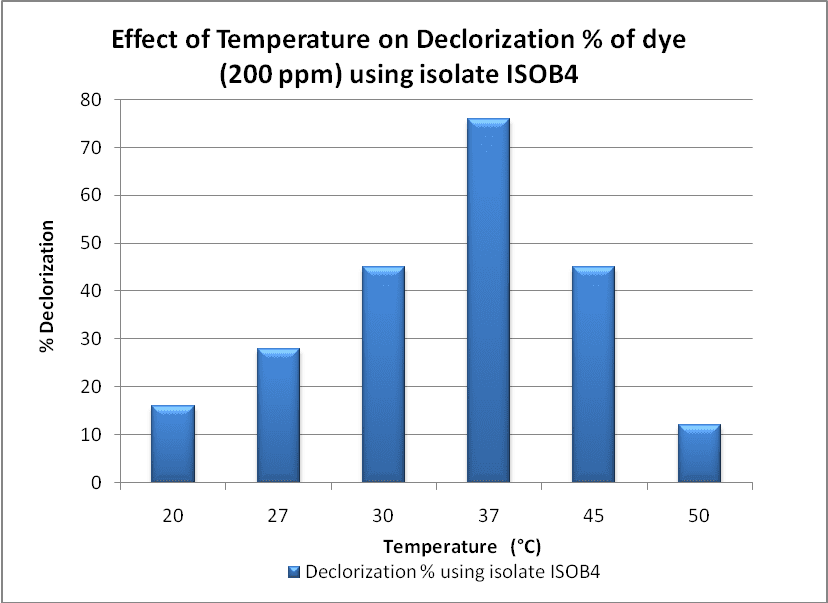
The pH and temperature are important factor for the optimal physiological performance of microbial cultures and decolorization of dyes. These factors affect the cell growth and various biochemical and enzymatic mechanisms. In present study the decolorization of Congo Red Dye by Potential isolate Bacillus sp (ISOB4) was found in the pH range of 3.0-11.0 [Fig:5,6]. The maximum decolorization (77%) was observed at pH 7.0. and at 37 0 C of temperature. A further increase or decrease in pH and temperature from the optimum value decreases the decolorization rate. It was found out that under agitation conditions, presence of oxygen deprives the azoreductase from obtaining electrons needed for cleavage of azo dyes [7].
Figure 6: Effect of Temperature on Declorization % of dye (200 mg/l) using isolate ISOB4
- Conclusion:
This study addresses the physicochemical characteristics of the effluents and the result revealed that the most of the parameters were not within the permissible limit of CPCB. Although decolorization is a challenging process to both the textile industry and the wastewater treatment, the results of this finding suggest a great potential for bacteria to be used to remove color from dye effluents. The textile dye is degradable with a concerted effort of bacteria isolated from an effluent disposal site. Further, it can be suggested that the potential of the bacteria need to be demonstrated in its application for treatment of dye bearing waste water using appropriate practice and through biotechnological approaches to color removal on one hand and on another hand Government regulation standards should more stringent regarding the removal of dyes from industrial effluents. Moreover, further research on these strains could explore new tools and techniques to evolve viable and eco friendly microbial solutions for treatment of dyeing industrial effluent.
Table 1: Biochemical and Morphological characteristics of isolates
| Sr. No. | Character | ISOB1 | ISOB2 | ISOB3 | ISOB4 | ||||
| Acid | Gas | Acid | Gas | Acid | Gas | Acid | Gas | ||
| A | Sugar fermentation | ||||||||
| 1. | Glucose | + | + | + | – | + | + | + | + |
| 2. | Sucrose | + | + | + | – | – | – | + | + |
| 3. | Lactose | + | + | + | – | – | – | + | + |
| 4. | Mannitol | + | + | + | + | – | – | + | + |
| B | Indole | – | – | – | – | ||||
| C | Methyl Red | – | + | – | – | ||||
| D | Voges-Proskauer | + | + | – | – | ||||
| E | Citrate | + | – | D | – | ||||
| F | H2S Production | – | – | – | – | ||||
| G | Catalase | + | + | + | – | ||||
| H | Urease | + (late) | D | D | – | ||||
| I | Gram staining | -ve | +ve | -ve | +ve | ||||
| J | Motility
| -ve | -ve | +ve | +ve | ||||
Key: -ve : negative ; +ve : positive ; D : differential
References:
- Khataee A.R. and Kasiri M.B., Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes, Journal of Molecular Catalysis A: Chemical 2010; 328: 8–26.
- Al-Kdasi .A, Idris .A, Saed .K, Guan .C, Treatment of Textile Wastewater by Advanced Oxidation Processes-A Review, Global Nest Int.J. 2004; 6: 222-230.
- AEPA (Australian Environmental Protection Authority, 1998), Environmental guidelines for the textile dyeing and finishing industry, State government of Victoria, Melbourne, Victoria, Australia.
- Dae-Hee A., Won-Seok C. and Tai-Il Y., (1999), Dyestuff wastewater treatment using chemical oxidation, physical adsorption and fixed bed biofilm process, Process Biochemistry, 34, 429–439.
- APHA, Standard methods for the examination of water and wastewater, 20th edn., “American Public Health Association”, Washington, DC, 2002.
- CPCB, Pollution control, acts, rules and modifications issued their under Central Pollution Control Board, New Delhi, 1995.
- Giwa, A., Akpan, U.G., Nkeonye, P.O., Bello, K.A., Kolawole, E.G. (2011). Solar photocatalytic degradation of acid blue 29. Journal of the Chemical Society of Nigeria, 36, 82 – 17.
- Rao A. V., Jain B. L. and Gupta I. C., Impact of textile Industrial effluents on agricultural land – A case study, Indian J. Environ Health, 1993; 35, No.2: 132 – 138.
- Abraha K., Gebrekidan A., Weldegebriel Y., Hadera A., Physico-Chemical Analysis of Almeda Textile Industry Effluents in Tigray, Northern Ethiopia, J Environ Anal Chem 2014; 1: 103.
- Daneshvar N, Ayazloo M, Khataee AR, Pourhassan M. (2007). Biological Decolorization of dye solution containing Malachite Green by Microalgae Cosmarium sp. BioresoU/: Techno 98: I176.
- Agarwal SK, Industrial Environment (Assessment and Strategy), APH Publishing Corporation, New Delhi, 1996; 276.
- Hu T L, Decolourization of reactive azo dyes by transformation with Pseudomonas luteola, Biores Technol, 49 (1994) 47-51.