Abstract
Vectran is a polyester (polyacrylate) fibre made from polymer with a high degree of crystallinity. It is a second-generation super fibre that followed Kevlar® and Twaron®, which are referred to as the first generation of super fibres. Vectran is the latest fibre discovered so its waste management is to be analysed before the waste becomes unbearable. The polymer is mostly used in composites so separation and reclamation are needed to be developed. This paper is a review of various reclamation process available and the future possibilities.
Introduction
Liquid crystal polymer may be a thermoplastic polymer from aligned molecule chains with spatial regularity of rigid crystal [1]. Affording to the situations during which the liquid is made, liquid polymer is often parted as thermotropic and lyotropic. &e first is by dissolving the polymer with a given solvent to realize lyotropic liquid-crystal polymers, while the second may be a thermotropic liquid crystalline polymer, when the liquid crystalline nature is possessing a thermal change under cooling and heating at a given temperature range is thanks to the chemical nature of polymer. Vectran as HM-HT fiber possesses strength and modulus a minimum of 2 GPa and 50 GPa, respectively [2]. Vectran which is fully aromatic polyester is commercially produced within the melt spinning method and it’s the sole thermotropic liquid crystalline polymer used on a billboard scale. liquid is thanks to some molecular motion matching with liquid and ordered crystal. Vectran forms a liquid crystal; due to an interactive and rigid polymer, it can easily be oriented through a fiber manufacturing process [3]. Vectran fiber is obtained from the dried then melted pellet thermotropic liquid crystalline polymer by melt spinning process and when it passes through a spinning hole it gives a high molecular orientation although; and by controlled cooling and solidification the structure of the fiber can be achieved. An excellent molecular orientation achieved during spinning causes an unnecessary post drawing process [4]. Production of Vectran is named cost-effective since it’s a melt-spun fiber than other high-performance fibres like aramids and UHMWPE; however, the need of high heat treatment for solid-state polymerization after melt-spun can offset its economical approaches. Vectran fiber application includes a high-performance purpose such as aerospace, military, and industrial applications [5].
Technology for Making Fibre from Thermotropic Liquid Crystal Polymer
The following part evaluates the characteristics of the Vectran polymer and the production process.
- Thermotropic liquid crystal polymers
Vectran was developed by applying the second methodology. The liquid polymer formed from the molecular structure that’s, the thermotropic liquid polymer obtained through the melt polymerization of p-Hydroxy carboxylic acid (HBA) and 2-Hydroxy-6-Naphthoic Acid (HNA)—was became fiber by melt spinning. The molecular chain of this liquid crystalline polymer features a high degree of orientation within the direction of the fiber length, which provides it excellent physical properties [6].
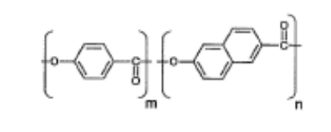
Figure 1. Molecular structure of Vectran’s main polymer

Figure 2. Diagrams of fibre structures.
- Creating fibers from thermotropic liquid crystal polymers
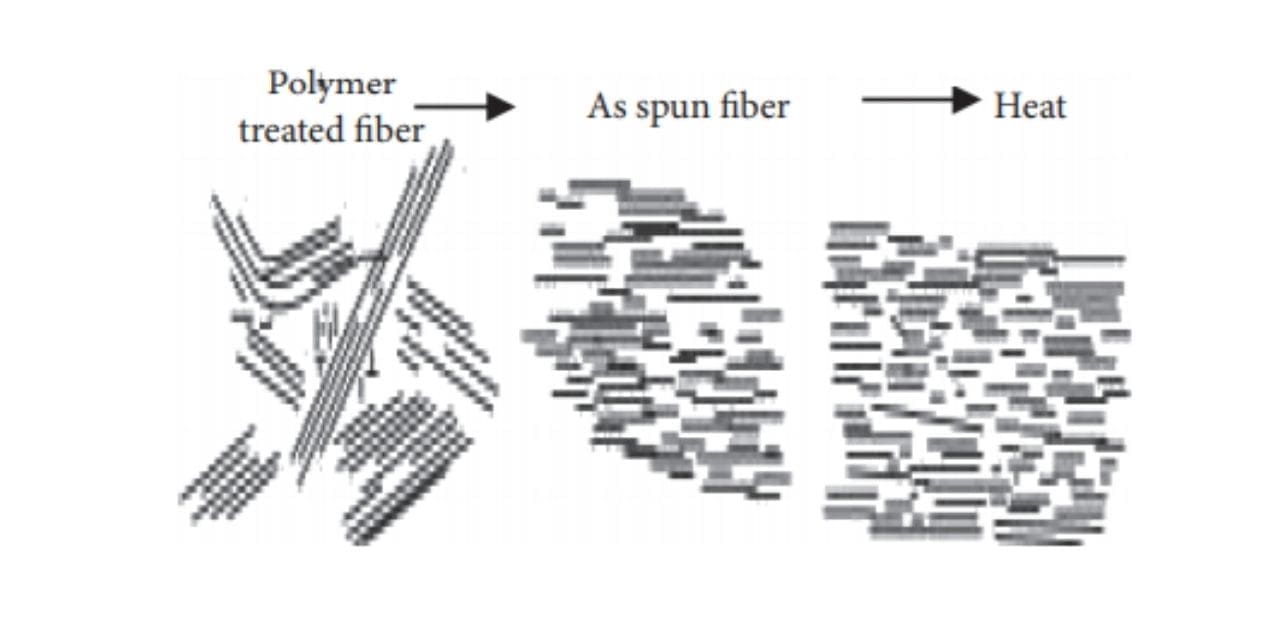
To turn thermotropic liquid polymers into fibers, pelletized polymer is first dried, then, after being melted in an extruder, it’s became fibers using the melt spinning method [7]. When thermotropic liquid polymer is spun under high shear stress, a high degree of molecular orientation takes place when the fabric passes through nozzles; this structure (orientation) is maintained until the fibers are cooled and solidify due to the lengthy time constant. If the fiber is ordinary polyester, it must be stretched to enhance its strength. On the opposite hand, because Vectran’s molecules are already during a rod-like and aligned state at the spinning phase, there’s no got to stretch the spun fibers again at a high draw ratio [8].
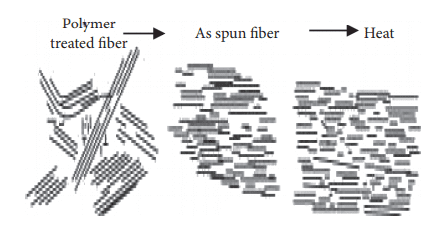
Figure 3. Fibre production concepts
- Heat-treatment
Although spun fiber already possesses considerable strength and coefficient of elasticity, which suggests stretching makes no sense for fiber formation because the molecular orientation has already been achieved within the spun state; heat treatment is performed to reinforce further performance [9]. The goal of warmth treatment is to extend the strength and coefficient of elasticity, and improve heat resistance. this is often achieved by continuously removing by-product materials in one among the subsequent conditions: (1) reduced-pressure; (2) noble gas atmosphere; and (3) active gas, including air atmosphere. Super fibers developed before Vectran were made by melting resin during a solvent (lyotropic liquid crystals); because solvents like vitriol were used, the manufacturing process was complex and dear. As Vectran is manufactured by the melt spinning method, this difficulty in the process is eliminated [10].
Need of Reclamation
The differences within the mechanical properties of conventional injection moulded plaques and pre-generated micro composites generated by using the twin extrusion process are studied for various TLCPs (Vectran) and matrices [11]. The pre-generated micro-composites are freed from skin-core morphologies that are present in injection moulded plaques. Therefore, the optimum processing method for generating in place TLCP (Vectran)/TP composites is that the dual extrusion process. Unfortunately, the high cost of TLCPs represents a drag only comparing this new sort of composites to composite systems that are reinforced with optical fiber, because Kevlar and carbon fiber are costlier [12]. Approximately the value of pure thermotropic liquid crystalline polymers ranges from $8.00 to $12.00 dollars a pound [13].
This is often quite like other traditional reinforcements utilized in thermoplastics as an example, carbon fiber is around $8.00 to $30.00 per pound and Kevlar fiber is around $15.00 per pound [14, 15]. However, with reference to the optical fiber utilized in composites, the value of the Vectran is far above the $1.00 per pound cost of this fiber [16]. Therefore, albeit TLCP / TP composites have excellent mechanical and physical properties, the general cost of the thermotropic liquid crystalline polymer / thermoplastic composites is substantially above the value of the glass filled composites. So, we can lower the cost of composite by recycling the TLCP and thus increasing the marketability. Many recycling methods believe merely grinding the composite blends then reprocessing the materials into further composite blends [17]. These methods would work for the injection moulded or the strand extruded composites, since these processes already involve compounding or mixing the materials together in one step. There has already been some work wiped out this area of reprocessing wholly thermoplastic composite strands by grinding up the recovered waste then reintroducing them back to the method [18]. They found that polypropylene reinforced with an undisclosed TLCP (Vectran) can undergo ten of those grinding / reprocessing cycles and see no loss in tensile strength. Unfortunately, this type of recycling would only work for composites, whose components have overlapping processing temperatures, and for composites that are processed within the same device.
In conclusion, the dual extrusion process produces the very best properties of composites generated with thermotropic liquid crystalline polymers and engineering thermoplastics. This process also eliminates the matter of morphological anisotropy or skin-core morphologies by producing continuous strands of TLCP fibrils within the composite fiber. Furthermore, by having the ability to extrude the 2 components separately, TLCPs and TPs with non-overlapping processing windows are often used and therefore the highest mechanical properties are often obtained.
Separation of Intimately Blended Species
In recent years, the world of mixed plastics recycling has seen a rise within the amount of research performed [19]. Intimate blending refers to a state during which, the dimensions of a pure introduce a mix is smaller than the dimensions of the littlest particle possible from mechanical grinding. Therefore, this part deals with the present technologies for the separation of heterogeneous blends.
- Mixed Plastics Recycling
In the area of recycling plastics, three methods are used to separate mixtures of polymers. The primary method of separation is named macro separation and it deals with separating large, pure components of polymers from one another. The second method of separation is named micro separation and it deals with the grinding of polymeric mixtures then using density differences to affect a separation. The third method of separation is named molecular separation and it deals with dissolving the polymer(s) then using temperature to cause a separation. For the macro separation method to be used, the mixture of polymeric pieces must contain large fractions of pure polymer within the mixed system [20]. Within the case of micro separation, since the respective polymers are adhered together, the pieces must be pre-processed within the sort of grinding. Once the materials are ground into sufficiently small pieces, the polymers are often separated using differences in density. This sort of separation relies on the principle that, though the polymers are adhered to every other, grinding of the materials leads to a distribution of particles that contain some pure components of every of the polymers present. The density is used to differentiate these pure components. However, this method only works if the polymers aren’t intimately blended [21].
- Selective Dissolution of Polymers
One of the new areas of separating mixed plastics is named molecular separation, and it deals with the utilization of a solvent to dissolve the polymers faraway from one another. Currently, this method is merely being studied on the tutorial scale thanks to the utilization of pricy and / or toxic solvents.
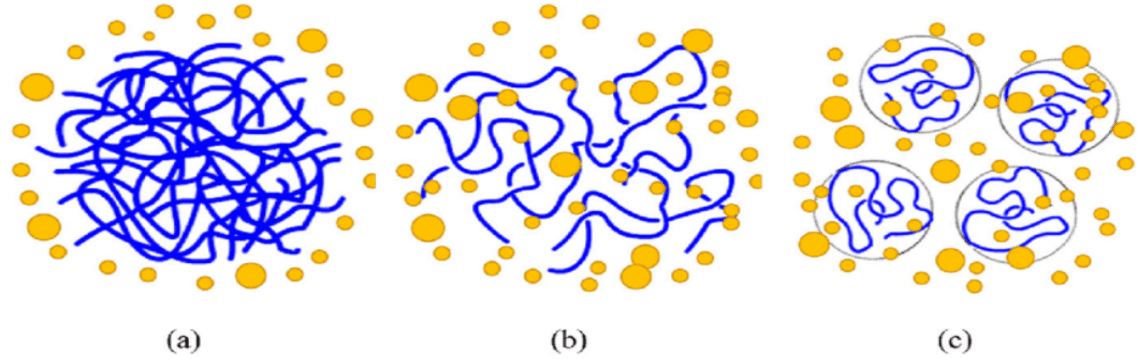
Figure 4: Diagrammatic representation of the dissolution process (25)
The Figure 4 shows the dissolution process of the polymer; blue lines represent polymer chains and yellow dots represent solvent molecules. (a) polymer in solid state just after added to a solvent; (b) a swollen polymeric gel; (c) solvated polymer dispersed into a solution.
There are two methods used for this sort of selective dissolution and separation method. the primary method involves using one solvent to dissolve all of the mixed polymers and therefore the second method involves using one solvent to only dissolve one polymer at a time. [22] The first major step within the molecular separation method is named selective dissolution, while the second major step is named flash devolatilization. The mixed polymeric material is ground up into very small pieces then sent to the separation unit. The separation unit, or selective dissolution step, consists of adding the polymeric mixture to a solvent then using the temperature to selectively dissolve certain components of the mixture. One among the polymers has been thoroughly and selectively dissolved, subsequent step is to filter the answer of non-dissolved contaminants then to blend in some stabilizers. Once the stabilizers are added, subsequent step is to require this solution to the flash devolatilization process. This process involves evaporating all of the solvent far away from the plastic and thereby leaving a pure, solvent-free polymeric material [23].
Conclusion
Vectran fibers is a synthetic fibre and are developed from a rigid-rod structure of TLCP. These works are concerned with developing an entirely thermoplastic composite which will compete with and / or replace traditional carbon or optical fiber composites. However, the cost of Vectran becomes the problem. The power to recycle or reclaim the thermotropic liquid crystalline polymer component (Vectran) of those wholly thermoplastic composites would be very cost effective. it might also make these composites more economically and environmentally attractive than the normal composites. Unfortunately, thanks to the relative youth of those pre-generated micro-composites, no work is often found that deals with the separation and reclamation of the Vectran component. So, the research during this field should be supported to seek out an answer to waste management beforehand.
References
| 1 | X. Wang, J. Engel, and C. Liu, “Liquid crystal polymer (LCP) for MEMS: Process and app,” Journal of Micromechanics and Microengineering, vol. 13, no. 5, pp. 628–633, 2003 |
| 2 | Y. Ji, Y. Bai, X. Liu, and K. Jia, “Progress of Liquid Crystal Polyester (LCP) for 5G Application,” Advanced Industrial and Engineering Polymer Research, vol. 3, 2020. |
| 3 | A. Blumstein, Liquid Crystalline Order in Polymers, Elsevier, Amsterdam, Netherlands, 2012. |
| 4 | K. Shaker and Y. Nawab, “Fibers for protective textiles,” in Fibers for Technical Textiles, Springer, Cham, Switzerland, 2020. |
| 5 | A. Greiner and H.-W. Schmidt, “Aromatic main-chain liquid crystalline polymers,” Handbook of Liquid Crystals, Wiley, Hoboken, NJ, USA, pp. 1–28, 2014. |
| 6 | H. Han and P. K. Bhowmik, “Wholly aromatic liquid-crystalline polyesters,” Progress in Polymer Science, vol. 22, no. 7, pp. 1431–1502, |
| 5 | S. Eichhorn, J. W. S. Hearle, M. Jaffe, and T. Kikutani, “Fundamentals and manufactured polymer fibres,” Handbook of Textile Fibre Structure, Elsevier, Amsterdam, Netherlands, 2009. |
| 6 | A. E. Zachariades and R. S. Porter, High Modulus Polymers: Approaches to Design and Development, CRC Press, Boca Raton, FL, USA, 2020. |
| 7 | S. Matsubara, H. Umetsu, T. Hase, and Toray Industries Inc, “Liquid crystalline polyester composition, method of producing the same and molded product manufactured from the same,” U.S. Patent 8.778.222, 2014. |
| 8 | J. Nakagawa, “Spinning of thermotropic liquid-crystal polymers,” Advanced Fiber Spinning Technology, Woodhead Publishing, Cambridge, United Kingdom, pp. 160–171, 1994. |
| 9 | J. Sarlin and P. Torma La, “Heat treatment studies of a TLCP ¨ fiber,” Journal of Applied Polymer Science, vol. 50, no. 7, pp. 1225–1231, 1993. |
| 10 | Y. Yamamoto and J. Nakagawa, Structure and properties of high-modulus, high-tenacity Vectran fibres,” in Handbook of Textile Fibre Structure, pp. 413–428, 2009. |
| 11 | J. D. Menczel, G. L. Collins, and S. K. Saw, “& Thermal analysis of Vectran fibers and films,” Journal of thermal Analysis, vol. 49, no. 1, pp. 201–208, 1997. |
| 12 | J. E. Taylor, A. Romo-Uribe, and M. R. Libera, “Molecular orientation gradients in thermotropic liquid crystalline fiber,” Polymers for Advanced Technologies, vol. 14, no. 9, pp. 595– 600, 2003. |
| 13 | Prescott, R., Modern Plastics Encyclopedia, McGraw Hill, New York (1992) |
| 14 | Pigliacampi, J.J., Modern Plastics Encyclopedia, McGraw Hill, New York (1992). |
| 15 | Anon, Modern Plastics Encyclopedia, McGraw Hill, New York (1992). |
| 16 | Leidner, J., Plastics Waste: Recovery of Economic Value, Marcel Dekker, Inc., New York (1981). |
| 17 | Sasaki, K. and Tomita, T., Kobunshi Ronbunshu, Japan. Journal of Poly. Sci. and Technology, 50, 11, 855 (1993). |
| 18 | Hegberg, B.A., Brenniman, G.R., and Hallenbeck, W.H., Mixed Plastics Recycling Technology, Noyes Data Corporation, Park Ridge, New Jersey (1992). |
| 19 | Lynch, J. and Nauman, E., “Separation of Commingled Plastics by Selective Dissolution”, Proceedings of Society of Plastics Engineers RETEC Conference- (1989) |
| 20 | S. Okamoto, M. Hirakawa, and Sumitomo Chemical Co Ltd, “Method for producing a liquid crystalline polyester and the liquid crystalline polyester,” U.S. Patent 7.009.026, 2006 |
| 21 | H. Avci, A. Hassani̇n, T. Hamouda, and A. Kiliç, “High performance fibers: a review on current state of art and future challenges,” vol. 27, no. 2, pp. 130–155, 2019. |
| 22 | K. Hori, Y. Hoshino, and H. Shimizu, Vectran: Development of High-Functionality Fiber and its Applications at Kuraray Co., Ltd 2014. |
| 23 | H. Hoshiro, R. Endo, and F. E. Sloan, “Vectran: super fiber from the thermotropic crystals of rigid-rod polymer,” in High Performance and Specialty Fibers, pp. 171–190, Springer, Tokyo, Japan, 2016. |
| 24 | S. Okamoto, M. Hirakawa, and Sumitomo Chemical Co Ltd, “Liquid crystalline polyester and method for producing the same,” U.S. Patent 6,512,079, 2003. |
| 25 | L. L. Chapoy, Recent Advances in Liquid Crystalline Polymers, Springer Science & Business Media, Berlin, Germany, 2012 |
Author:

M.Tech Student, Department of Fibres and Textile Processing Technology, ICT Mumbai.
GUIDE:
SUMAN MUNDKUR