Author and Co-Authors Name: Digambar Chavan, P V Kadole
DKTE’s Textile & Engineering Institute Ichalkaranji, India.
Abstract: Solid State Polymerization (SSP) is a process in which the polymer chain lengths are increased by heat in the absence of oxygen and water, by means of either vacuum or purging with an inert gas to drive off the by-products of reactions. The reaction is driven by temperature, pressure, and the diffusion of by-products from the interior of the pellet to the surface. SSP is an important step frequently used after melt-polymerization for the purpose of enhancing the mechanical and rheological properties of polymers before injection blow molding or extruding. The SSP technique is widely applied in industrial manufacture of bottle-grade PET, films, and superior industrial fibers.
Keyword: SSP- Solid State Polymerization, Tg Glass Transition temperature, Tm Melt temperature, IV Intransic Viscosity,
——————————————————————————————————
After polymerization in the melt phase, the molecular weight of polyester can be further increased by polycondensation of PET chips in the solid state and this process is called solid state polymerization (SSP). This process enables higher molecular weights to be reached which are either technically or commercially not feasible in the melt phase. Solid state polymerization in the form of chips or powder, pre-extrusion SSP is a common approach in the production of high molecular weight polymers for molded products.
Polycondensation reaction is exothermic, therefore, it can also be driven forward to give polymer with higher molecular weight if the reaction is carried out at a lower temperature. At lower temperatures of polycondensation, equilibrium shifts forward to higher completion enabling formation of higher molecular weight of the product. Since temperatures cannot be lowered in melt polymerization, the best solution is to carry out the reaction in solid state, wherein temperatures lower than melting point of the polymer can be used. At these temperatures, the kinetics of the reaction is slow and it takes a long time to complete the reaction.
The molecular weight can be increased up to 27000 (IV, 0.90) for bottle grade and as high as 38000 (IV, 1.20) for technical applications such as tyre cord, seal belts and air bags
The first step before carrying out the solid state polymerization is precrystallization of chips. The water quenched polymer chips obtained from melt polycondensation stage are almost wholly amorphous. Therefore, the chips are first intensely dried and annealed at a gradually increasing temperature up to the point of maximum crystallization rate to impart high level of crystallinity. This raises the glass transition temperature of the chips and prevents their sticking to each other during solid state polymerization.
The SSP is performed at temperatures between 220 and 235°C for PET, which lie above the glass transition temperature and below the crystalline melting point of PET. Under these conditions, polymer chain end groups are mobilized sufficiently to undergo polycondensation. The annealed chips are heated in a stream of hot inert gas or by agitating in a vacuum drier to remove small traces of EG and other volatile by-products. As the reaction is carried out in the solid phase, volatile by-products can diffuse easily to the surface of each polymer chip and are instantly carried away by the gas flow or high vacuum.
The main advantages of increasing molecular weight in solid state compared to melt phase are:
- Problems associated with stirring of viscous melt are eliminated.
- Degradation and side reactions are limited in the solid state due to lower processing temperature used. Solid state polycondensation process favours polymerization process compared to depolymerization.
- Best means of achieving PET with acceptable level of acetaldehyde.
The rate of the solid state polycondensation process depends on particle size, initial molecular weight, crystallinity of PET chips and end group concentration. The pellet size has generally been used to identify the rate controlling mechanism of overall reaction rate. When the reaction rate is chemically controlled, then the pellet size has no effect on the reaction rate. On the other hand, if reaction rate is diffusion controlled, i.e. limited by the diffusion of byproducts from inside of the pellet chips to the pellet surface, then the reaction rate can be increased by decreasing the pellet size, due to decrease in the length of diffusion path.
It has also been shown that the higher starting molecular weights enable higher molecular weights to be achieved. this has been attributed to the tendency of the lower starting molecular weights to lead to higher crystallinity build-up during SSP. Crystallization reduces the SSP reaction rate by reducing chain mobility and by increasing the diffusion path length of the by-products. The crystallinity of polymer should be between 33 and 58% and should not exceed 80%.The reaction rate is very sensitive to the ratio of hydroxyl to carboxyl end groups. At low carboxyl concentrations, the transesterification reaction will be favoured, while at high carboxyl concentrations the esterification route will be preferred. The optimum ratio of hydroxyl end groups to carboxylic end groups has been experimentally found to be two.
A.How the Process Works
- Receive PET, polyesters or other condensation polymers made by melt polymerization
- Transfer the pellets to a Rotary Vacuum Dryer (RVD)
- Generally, the vacuum pump removes air, water and by-products of the SSP; alternatively, nitrogen purging is used
- The temperature/time profile is controlled with a microprocessor
- Initial heating at a temperature above Tg cold crystallizes amorphous pellets to prevent sticking and fusion of pellets
- Heating is continued to dry the ”wet” pellets
- Heating is continued to a predetermined temperature below the melting temperature of the polymer
- Solid state polymerization is done at a high temperature and low vacuum (or alternatively at zero oxygen level with nitrogen purging) to achieve the desired high molecular weight of the polymer
- The SSP progress is followed by periodically taking samples with custom designed samplers
- The melt viscosity at a specific temperature of that sample is determined within several minutes
- The Intrinsic Viscosity is obtained from the Correlation Chart/Curve established from melt viscosity data and Intrinsic Viscosity obtained from solution viscosity at ambient temperatures for PET homopolymers
- The final product viscosity is as specified by the customer
- A major benefit of solid state polymerization is obtaining a desired high molecular weight polymer of uniform and consistent Intrinsic Viscosity
- Other major benefits include the reduction of undesirable side reactions and the levels of by-products
- For PET, the SSP by-products are ethylene glycol and acetaldehyde
- When the desired molecular weight is achieved, the product pellets are cooled and transferred to storage tanks
- The product pellets are classified, packaged and shipped according to customer requirements.
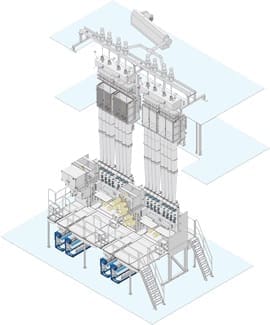
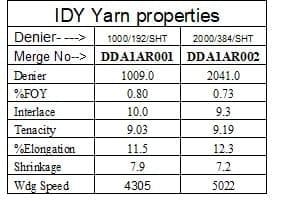
Applications of IDY:-
Automobile tires, safety belts, airbags, geotextiles, mooring ropes for drilling platforms, conveyor belts, sails and fishing nets – all these are manufactured using industrial yarns. Industrial yarns are considered to be the ultimate discipline in filament manufacturing. High tenacities, extreme dimensional stability, tremendous durability along with a large range of titers – although the demanding production process promises high-margins, it is however also simultaneously a huge challenge both for the yarn manufacturer and the systems constructor.
REFERENCES:-
[1]. Rabindranath K, Mashelkar R A, J. Appl. Poly. Sci. 1990.
[2]. Culbert B., Christel A. “Continuous Solid state Polymerization of Polyesters”.
[3]. Papaspyrides C.D, Vouyiouka S. N; “Fundamentals of Solid-state Polymerization.
[4]. petus.com/ssp.aspx
[5]. nptel.ac.in/courses/116102010/40