Chet Ram Meena, Neha Mehra & R.V. Adivarekar
Department of Fibres and Txtile Processing Technology,
I.C.T., Mumbai, India
Email: rv.adivarekar@ictmumbai.edu.in
- Introduction
In recent years there has been an increasing awareness about environmental friendliness in all human activities [1]. The textile industry is a water intensive industry with water being used in every stage of wet processing from sizing, desizing, scouring and bleaching of fibers to the dyeing, finishing and printing of fabrics. Every textile plant requires large volumes of water and produces high volumes of effluent wastewater. The typical textile dye wastewater composition is quite complex. The demand for environmental friendly dyes and application processes is therefore very strong [2].
Reactive dyes have become very popular for cotton due to its brilliancy, variety of hue, high wet fastness, convenient usage and high applicability [3].
Reactive dyes are anionic in character and cotton fibers also adopt anionic surface charge in water causing limited exhaustion of dye due to charge repulsion. Large quantities of electrolyte (30-100 g/l) are thus added to overcome this problem. One of the major problem of reactive dyeing is the large amount of electrolyte required for exhaust and pad application [4] which leads to environmental problem. In addition, inadequate dye exhaustion and fixation result in coloured effluents. As environmental problems arising from dyeing with reactive dyes have become critical, many studies have been devoted to improving the substantivity of cotton fiber for reactive dyes, thus reducing or eliminating the amount of electrolyte used.
The problem has many solutions of which cationic reactive dyes and the modification of cotton by cationisation with various cationising agents seems to be most promising [5].
In this paper, we have reviewed problems of pollution due to electrolytes in reactive dyeing and finding a solution through cationic reactive dyes. The agenda of this review paper is to show that cationic reactive dyes are more suitable than conventional reactive dyes wherein there is a possibility of elimination of electrolyte with enhancement of the fabric fastness properties.
2.Problems caused by use of Electrolytes in Reactive Dyeing
- Impairs the delicate biochemistry of aquatic organism.
- If sodium sulphate is used as electrolyte, due to the formation of alumina-sulphato complexes which swell and crack concretes with considerable alumina content there is a destructive attack on the concrete pipes.
- Evolution of hydrogen sulphide gas under anaerobic conditions when sodium sulphate is used as electrolyte. Dissolution of such sulphides and subsequent bacterial oxidation to the harmful sulphuric acid.
- Increase of the TDS of the effluent [6, 7].
3.Approaches for Electrolyte-free Dyeing
The two approaches by which a solution for the problems caused due to the use of electrolytes in reactive dyeing of cotton and its blends can be as follows:
- Cationic Reactive Dyes
- Modification of Cotton by Cationization
3.1 Cationic Reactive Dyes
An important factor in reactive dyeing is that of the electrostatic repulsion between the sulphonic acid-containing reactive dyes & the surface of the fibre. Another kind of electrostatic repulsion operating is again that between the fixed dye & the incoming dye. Thus, to increase the exhaustion of the dye one needs to employ a high concentration of electrolyte in the dye bath.
Among various remedies to this, the concept of having a cationic reactive dye in place of anionic reactive dyes has been suggested as an effective solution in recent years[8].
3.2 Modification of Cotton by Cationization
Cellulose fibers when immersed in water produce a negative zeta potential and most of the dye classes suitable for cotton are anionic in nature. The negative charge on the fiber repels the anionic dye ions and consequently the exhaustion of the dye bath is limited. However this zeta potential can be easily offset by salt concentrations of a few ppm, about 10 – 100ppm [9].
Cationic cotton is cotton that is modified to contain a quaternary group. Such cationic cotton has an enhanced affinity for anionic dyes. This is because when cotton is cationized with a reactive type of quaternary ammonium compound it forms an integral part of the cellulose chain [10].
Cationization of cotton for dyeing is a long process as it involves cationisation of the fabric in the first stage followed by dyeing in the second stage. Also, this increases the overall consumption of water and effluent load and as complete exhaustion of cationizing agent does not take place, small quantity of the cationizing agent is also released. Also, if cationization process does not take place uniformly or crease formation takes place, the following dyeing obtained would be non-uniform. Hence in comparison, cationization of cotton process is time consuming, giving more effluent with the cationizing agent as compared to the alternative process i.e. dyeing using cationic reactive dye which is a single bath process so of shorter time and thus no additional effluent load being produced.
4. Applications of Cationic Reactive Dyes
Cationic reactive dyes can be applied for the following fibres.
4.1 Cationic Reactive Dyes for Cellulosic Fibres
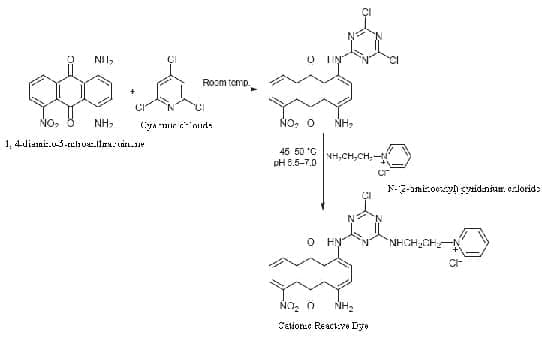
The dyeing of cellulose requires dyes that are in a water soluble form due to the hydrophilic nature of the cellulosic fibres. But due to anionic dye and fibre repulsion as discussed earlier, the dyeing method requires the presence of electrolytes which suppress negative charge build-up at the fibre surface and promotes increased dye adsorption. An alternative approach to improving dyeability of cellulose with anionic dyes could be achieved by incorporating cationic groups into the cellulose structure. Cationic dye ranges are not yet practically used for the dyeing of cellulosic fibre, even though they offer the possibility of a salt-free dyeing process with high tinctorial strength at relatively low levels of dye application. By considering localised cationic dyes where quaternary ammonium groups attach to the chromophore via an aliphatic spacer linkage, it is speculated that removal of these quaternary ammonium groups after the dyeing process may be possible without causing a significant shift in colour hue [11]. The long alkyl chain quaternary ammonium salts (QAS) structures are incorporated into reactive dyes as both water-soluble and cellulosic fiber interactive groups. The QAS are water soluble and more importantly carries positive charge in water, which will increase dye interaction with negatively charged cellulosic surfaces. Thus, the reactive dye molecule can assist dye exhaustion onto cellulosic fibers without using salts and possibly increase reactivity between the cellulose and the dye. This consequently reduces potential hydrolysis of the reactive dyes and the dyed cotton exhibits good color washfastness [7].
Figure 1: An illustrative example of synthesis of cationic reactive dye [11]
4.1.1 Dyeing Procedure
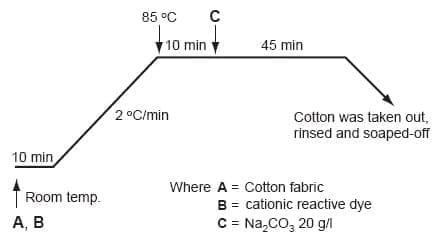
Cotton fabric can be dyed by cationic reactive dye as per the procedure described in Figure 2. Initially the fabric and dye need to be rotated at room temperature followed by raising the temperature to 85oC. At this temperature sodium carbonate needs to be added for fixation to take place. The dyeing can then be continued for another 45mins and removed followed by soaping using non-ionic surfactant and sodium bicarbonate.
Figure 2: Dyeing procedure of the cationic reactive dye [11]
4.2 Cationic Reactive Dyes for Protein Fibres
Reactive dyes are widely used for dyeing and printing protein fibres. The hydrophilic group of conventional reactive dyes is an anionic group, e.g. sulphonate or carboxylate, but the hydrophilic group of reactive cationic dyes is cationic. The general formula for this is given in Figure 3.
R-D-N+R3’
Figure 3: Hydrophilic group of reactive cationic dyes, where R is the reactive group,
D the chromophore and N+R’, the cationic group.
The reactive dye molecules can react with wool fibre under suitable conditions because of their reactive group (monofluorotriazene). In general, the higher the pH value of the dyeing solution, the more easily the nucleophilic substitution reaction takes place. Because wool fibre has an isoelectric point, the higher the pH value, the more free amino groups are available. The surface of fibre is electronegative and the dye ion is positively charged, so it is easy for the latter to be adsorbed and to react. Cationic reactive dyes are quite different from conventional reactive dyes in dyeing protein fibre. The presence of anionic groups in conventional reactive dyes means they tend not to be adsorbed [12].
4.3 Cationic Reactive Dyes for Synthetic Polyamide Fibres
Dyes for polyamide fibres normally form ionic bonds within the polymer matrix. In this dyes bearing a negative (anionic) charge are used because polyamides carry a positive (cationic) charge – especially during the dyeing process [13].
Dye-SO–3 Na+ + Nylon-NH3+ Cl– à Dye-SO3– +H3N-Nylon
Figure 4: Schematic representation of dye–polymer binding via ionic bonding on Nylon
Anionic (acid) dyes are commonly used to colour nylon. The dye attaches to nylon via electrostatic linkages between the cationic, protonated amino end groups of the nylon (NH3+) and the anionic sulphonate groups of the dye (Dye-SO3_) [14].
However, even fabrics coloured with these dyes suffer colour loss during laundering which causes staining of adjacent fabrics. An after treatment of the dyed nylon can result in somewhat improved wet fastness, repeated washing can still result in loss of colour and there remains much room for improvement. Reactive dyes resemble acid dyes in their basic structure, but additionally possess fibre reactive groups, their name being derived from their ability to react chemically with the fibre.
Cationic reactive dyes possess either a single mono- or dichlorotriazine reactive group or a heterobifunctional (monochlorotriazine ⁄ sulphato ethylsulphone) reactive system, containing one or two cationic trimethylammonium ((CH3)3N+) groups, which get attracted to the negative carboxylate groups (COO–) of nylon via ion–ion attraction. The optimum pH of application is 8–10. These conditions provide the best balance of electrostatic attraction between dye and fibre, together with a high concentration of free (nucleophilic) amino groups on the fibre. These cationic reactive dyes exhibit very good build-up and fixation efficiency, comparable with anionic monochlorotriazine commercial reactive dyes for nylon [15]. However, mild staining of polyamide and secondary cellulose acetate occurs in some cases wherein mild acidic soaping-off treatment can reduce the staining [16].
A high pH is used because at that pH the build up and fixation of anionic reactive dyes on nylon is limited by electrostatic repulsion between dye and anionic carboxylate groups present in the nylon. At low pH, the effective concentration of anionic carboxylate groups is greatly reduced, and that of cationic protonated amino groups increased, leading to electrostatic attraction between dye and fibre but a massive reduction in the concentration of free amino groups which are the nucleophilic species responsible for reacting with dye. Under alkaline conditions the fixation and build up of cationic reactive dyes on nylon are excellent. Also, because covalent bond formation between dye and nylon is efficient, dyeing shows excellent wet fastness [17].
4.4 Cationic Reactive Disperse Dyes for Multifibres
The present method of dyeing multifiber fabrics such as polyester/wool/CDP (Cationic Dyeable Polyester), polyester/viscose/CDP blends involves the use of various two-bath processes, disperse dyes for polyester, reactive dyes for wool or viscose, cationic dyes for CDP using a lot of disperse agents. The use of various multi-bath processes is time-consuming and expensive to operate. The effluent pollution from dyeing bath containing dispersing agents and leveling agent is large, having a high biological oxygen demand (BOD). The temporarily solubilised cationic reactive disperse dyes containing pyridine-quaternary group have been designed for dyeing of multifiber fabric. As heterocyclic nitrogen atoms of pyridine cause an electron deficiency at the adjacent carbon atom, the dyes containing pyridine-acetylamino are susceptible to react with natural fiber. These dyes are temporarily solubilised disperse dyes. After hydrolyzing, they become disperse dyes for PET. The dyes containing quaternary group can be used for one-bath processes with cationic dyes without dispersing agents and levelling agent for polyester/wool/CDP and polyester/ viscose/CDP.
Cationic reactive disperse dyes shows good degree of exhaustion and fixation on wool. The light fastness, rubbing and wash fastness on wool is excellent as compared to any conventional reactive dyes [18].
6 Conclusion
To put it in a nut shell, the main problem faced by dyeing industry is due to electrolytes used in dyeing. The cationic reactive dye can be looked upon as a solution to this problem as cationic reactive dyes shows high percentage exhaustion and fixation value, in the absence of electrolyte. None the less, the tailor made cationic reactive dyes can be applied on almost all commercially used fibres. Fastness properties are considerably good compared to the conventional reactive dyes. Thus, cationic reactive dyes can offer not only simpler dyeing recipes but also possible environmental benefits.
- References
- N. Sekar, Colourage, pp 93-94, December 2001.
- Environmental Protection Agency, Development Document for Effluent Limitations Guidelines and Standards for the Textile Mills; Points Source Category, EPA Document 440/1 – 79/ 022b, EPA, October 1979.
- Zhang jie., Dyeing and Printing, 4, pp 47-50, 2005.
- Haigh D., Review of Process in Coloration, 2, pp 27, 1971.
- Jocic, D., Jovancic, P., Petrovic, Z. Lj., Bertran, E., Navarro, A., Julia, M. R., & Erra, P., In Proceedings of the World Textile Conference 2nd AUTEX Conference. Bruges, Belgium, 2002. 297–312.
- M. Subramanian, Journal of Textile Apparel Technology and Management, 5 (2), pp 1-16, 2006.
- Tao Zhao, Gang Sun, Xinyuan Song, Journal of Applied Polymer Science, 108, pp1917–1923, 2008.
- N. Sekar, Colourage, pp 47-49, March 2001.
- Rakesh V. Tiwari, Colourage Special, Supplement on BTRA, Colourage Seminar on Reactive dyes, 28 Sept., 38 (9), pp 19 – 22, 1991.
- R.B. Chavan, and D.P. Chattopadhyay, Colourage Annual, 45, pp 127 – 133, 1998.
- Kawee Srikulkit and Pornchai Santifuengkul, JSDC, 116, pp398-402, 2000.
- X. Kongliang, H. Aiqin, Journal of Society Dyers and Colourist, 114, pp 20-23, January 1998.
- https://monographs.iarc.fr/ENG/Monographs/vol99/mono99-7.pdf, assessed on 18 November 2011.
- A. Soleimani-Gorgani1, J.A. Taylor, Dyes and Pigments, 76, pp 610-623, 2008.
- Atasheh Soleimani-Gorgani, John A Taylor, Coloration Technology, 127, pp1–8, 2011.
- D. M. Lewis, L. J. Sun, Coloration Technology, 119, pp 327- 330, 2003.
- A. Soleimani-Gorgani1, J.A. Taylor, Dyes and Pigments, 76, pp 610-623, 2008.
- Kongliang Xie, Aiqin Hou, Journal of Dispersion Science and Technology, 29, pp 436–439, 2008.