1 Terminologies
1.1 Fibre
The unit of matter characterised by flexibility, fineness and high length to diameter ratio (For textile grade fibre length to diameter ration is 1000: 1.
1.2 Yarn
The yarn is an assembly of fibre twisted/bound together to form a continuous strand.
1.2.1 Staple yarn
The yarn made of out of fibre which has limited length.
1.2.2 Filament yarn
It is the name given to a fibre of continuous length.
1.3 Monomer
A single unit in the polymer is called as a monomer.
1.4 Polymer
Monomer Many monomer molecules Have linked together to form a polymer (molecule).
1.4.1 Types of polymerization
Addition polymerization
With this type of polymerization, the monomers add or join end-to-end without liberating any by-product on polymerization. Examples of fibres consisting of addition polymers are acrylic, modacrylic, polyethylene or polyethene, polypropylene or polypropene, poly-vinyl alcohol (PVA) and the chlorofibres, namely polyvinyl chloride and polyvinylidene chloride.
Example:
CH2=CH2 + CH2 = CH2 + CH2=CH2 + …
-CH2-CH2-CH2-CH2-CH2-CH2 –
(Ethylene) (Polyethylene)
Condensation polymerization
With this type of polymerization, the monomers join end-to-end and liberate a by-product. This by-product is usually a simple compound- generally water, but may be hydrogen chloride or ammonia, depending upon the specific monomers. Examples of fibres consisting of condensation polymers are elastomeric, nylon and polyester.
Example:
HO(CH2)5COOH + HO(CH2)5COOH
HO(CH2)5CO.O(CH2)5COOH + H20
(Hydroxy acid)
1.4.2 Types of polymer
Linear polymer
The growth of the polymer takes place in only length-wise direction.
Branched polymer
The growth of the polymer takes place in two directions e.g. Plastic sheet.
Cross-linked polymer (3D polymer)
The growth of the polymer takes place in three directions. Linear polymer is suitable for the production of fibre.
1.5 Thermoplastic
When appropriately heated, the thermoplastics melt or fuse and exhibit continuous flow preferably under pressure and most of them can be dissolved in appropriate solvents. Cellulose acetate rayon, nylons, vinyl chloride- or vinylidene chloride-vinyl chloride copolymers, glass fibre etc. are examples of thermoplastics.
1.6 Non-thermoplastic
Non-thermoplastic begin to decompose on heating before exhibiting plastic flow and they can be seldom dissolved in solvents. All natural fibres together with viscose and cuprammonium rayons (regenerated cellulose), wool and asbestos fall in this category.
1.7 Degree of polymerization (n)
A number of the repeating unit of the monomer is called as the degree of polymerization.
The length of the polymer is most important. All fibres, both man-made and natural, have long to extremely long polymers. The length of a polymer can be obtained by determining its degree of polymerization. This is often abbreviated OP and defined by the following mathematical expression:
D.P. =(Average molecular weight of polymer)/(Molecular weight of the repeating unit in the polymer)
1.8 Molecular weight of fibre
Molecular weight is the sum of atomic mass of each constituent and the molecular weight of fibre can be given as follows:
Molecular weight of fibre = Molecular weight of mer unit ×Degree of Polymerization
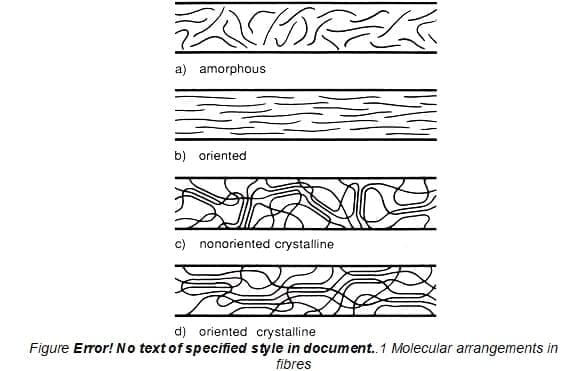
1.9 Orientation
The alignment of the long molecular chain. relative to the fibre axis is called orientation.
1.10 Crystalline region
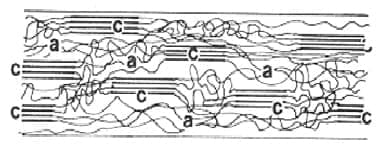
It is the region where molecules are regularly and closely packed. In this constituent elements/ molecules have fixed positions in space relative to each other. This accounts for the solid structural rigidity. It gives stiffness to the structure. Gives durability to the fibre structure. It gives strength to the fibre. If any fibre is having higher crystalline region then that fibre is stronger, more durable, less easily degraded by chemicals, less plastic, resist being distorted, less absorbent as well as pliable and stiffer handle.
Figure 1.1 crystalline and amorphous regions in molecular structure of fibre a = Amorphous, c = Crystalline
 1.11 Amorphous region
1.11 Amorphous region
It is the region of fibre structure where molecular chains are random and loses bonded. It gives flexibility, an extension to the fibre. This region is responsible for absorption of moisture and dye. If any fibre is having higher amorphous region then that fibre is more absorbent, can be dyed easily, more pliable, softer in the handle, weaker, has less durability, plastic, and can easily distort easily degraded by chemicals.
1.12 Molecular structure
The spatial arrangement of atoms within the molecule or configuration.
1.13 Supermolecular structure
The arrangement of the molecules in a condensed state and the formation of crystallites.
1.14 Morphology
The arrangement of crystallites in the fibre.