Abstract
Polyurethanes (PUs) are a special group of polymeric materials that are in many ways different from most of the other plastic types. They can be incorporated into many different items, such as paints, liquid coatings, elastomers, insulators, elastic fibers, foams, integral skins, etc. The invention of the diisocyanate polyaddition technique by these researchers led to the creation of the PU industry in 1937 by Otto Bayer in Germany, with PU produced through the reaction between diisocyanate and polyester diol PU structure is usually determined by hard and soft segments, molecular weight, polydispersity, and crosslinking ability.
Water based polyurethanes dispersions (PUDs) are one of the most growing segment for the surface coating industry due to their technological advances, that has made them an effective substitutes for the solvent based analogs. Water based polyurethanes dispersion for manufacture of adhesive has excellent performance on heat resistant, fast drying, endurance bonding strength, easy to water clean –up, excellent adhesion, flexible and atomization.
Specifically, PUs find wide application in coatings due to their specific properties, such as their excellent mechanical strength, toughness, good abrasion, corrosion, good adhesion, chemical resistance and low-temperature flexibility. Soft film will be formed and very suitable for coating on the soft materials, have cross linking reaction with ethylene amine and polycarbodimide.
Introduction
Coatings and adhesives that make use of water primarily as the solvent are often referred to as waterborne polyurethanes (WPUs). There are several pieces of legislation that place restrictions on the amount of allowed volatile organic solvents and other hazardous air pollutants that may be released into the environment. Most commercial and industrial applications are therefore dependent on polyurethane dispersions (PUDs), or waterborne polyurethane dispersions (WPUDs). PUDs have the unique advantage that the viscosity of the dispersion is not dependent on the molecular weight of the polymer. Therefore, high solid-content WPUs (HSCWPUs) can be prepared by the drying process only.
Waterborne polyurethane dispersions (PUD) are fully-reacted polyurethane systems produced as small discrete particles, 0.1 to 3.0 micron, dispersed in water to provide a product that is both chemically and colloidally stable, which only contains minor amounts of solvents and thus emit very little volatile organic compounds. Waterborne PUDs are based on aliphatic – IPDI or H12MDI – or aromatic – MDI or TDI – isocyanates, modified polyether and/or polyester polyols, chain extenders, catalysts plus additives to modify the coalescence, flow, thickness, coagulation and defoaming properties.
Waterborne PUD is produced in conventional stirred reactor fitted with distillation equipment. The first step in the manufacture of an anionically-stabilised PUD is to prepare a prepolymer from isocyanate, polyol (containing either carboxylate or sulpfonate side chains) and chain extenders in a water-miscible solvent such as acetone.
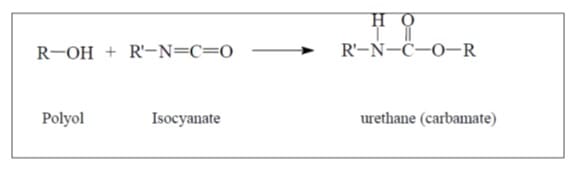
Figure: Reaction of the polyol and isosynate
The reaction product is an isocyanate-terminated polyurethane or polyurea with pendent carboxylate or sulpfonate groups. These groups can be converted to salts by adding a tertiary amine compound, which, as water is added to the prepolymer/solvent solution, disperses the prepolymer in the water.
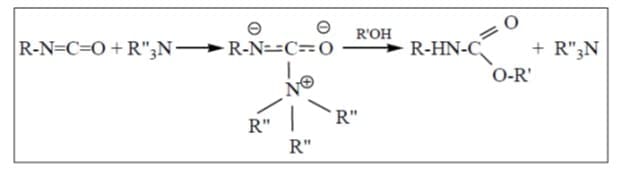
Figure: Amine catalyst reaction mechanisms
Classification of water based PUDs
Based on the ionic charge that a polymer molecule carries on it, PUDs have been classified into three categories viz. anionic, cationic and non-ionics.
Anionic PUDs
They are stable at alkaline pH values > 7. Usually commercial PUDs are anionically stabilized, since the dispersing agent is normally a bishydroxy carbvoxylic acid. These are PUDs in which part of the polyol component is replaced by a monomer containing pendant carboxylic acid or sulfonic acid groups.
Cationic PUDs
They are stable at acid pH (<7) and are normally based upon alkylated or protonated tertiary amines. These PU ionomers are prepared in similar fashion but incorporate monomers containing a tertiary amine group. The ionic centers are formed by protonation with strong acids or by quaternization with alkylating agents.
Non-Ionic PUDs
Non – Ionics have no polarity (non-ionisable) and are stable over a very wide pH range. These are grades in which the ionic centres are replaced with hydrophilic polyether units, either branching off or terminating on the main PU chains. Polyethylene oxide units (MW 200–4000) are normally used as the dispersing sites.
Types of PUD Systems
1-K Polyurethane Dispersions
1K PUDs are difficult to synthesize and formulate and also pose several application restrictions though they may offer superior properties as compared to 2K PUDs. These special breed of PUDs have been developed quite recently and have not yet found much industrial significance due to their high manufacturing costs.
2K Polyurethane Dispersions
Two component systems offer excellent finishes, very good hardness, external appearance and chemical resistance. In this breed of PUDs, hydroxyl functional polyurethane resins which can be crosslinked with HDI trimers or biurets are genereally used in prepolymerization process.
Various Methods of Making Polyurethane Dispersions
- Emulsifier-Containing Dispersions
- Ionomer Dispersions
- Non-Ionic Dispersion
Ingredients for Waterborne Polyurethane Dispersions
- Isocyanates Crosslinkers
Aromatic isocyanates:
- Methylene diphenyl diisocyanate (MDI),
- Toluene diisocyanate (TDI) and
- 1,5-Naphthalenediisocyanate (NDI)
Aliphatic isocyanates:
- Hexamethylene diisocyanate (HDI),
- Isophorone diisocyanate (IPDI) and
- 4,4’-Diisocyanatodicyclohexylmethane (H12MDI)
The catalysts are used to speed up the reaction between the isocyanate and polyols and to allow reaction at a lower reaction temperature. Most often, catalysts are used in the formulation of different kinds of PUs for selective purposes. Catalysis plays a vital role in the preparation of urethane and ureathane-urea polymers, because it not only affects the rates of the chemical reactions responsible for chain propagation, extension, and cross-linking but also affects the ultimate properties of the resulting polymers. Catalysts are employed whose functions are not only to bring about faster rate of reaction but also to establish a proper balance between the chain-propagation reaction (primarily the hydroxyl-isocyanate reaction) and the foaming reaction.
Neutralizing Agents
The neutralizing component consists of one or more bases which serve for neutralizing some or all of the carboxyl and/or sulfo groups. For example, tertiary amines, such as N,N-Dimethylethanolamine, N-Methyldiethanolamine, triethanolamine, N,N-Dimethylisopropanolamine, N-Methyldiisopropanolamine, triisopropanolamine, N-Methylmorpholine, N-Ethylmorpholine, triethylamine or ammonia, or alkali metal hydroxides, such as lithium hydroxide, sodium hydroxide, potassium hydroxide or mixtures thereof, can be used as suitable bases. Tertiary amines and in particular triethylamine are preferably used.
Dimethylolpropionic Acid
Dimethyopropinonic acid is a main raw material for manufacturing water soluble polyurethane; presently, DMPA has been widely applied to the production of emulsified coating agent for leather. Besides, it can be applied to the manufacturing of polyester dope, photosynthetic substance, liquid crystal of new type, adhesive and magnetic recording materials etc. Adding DMPA can improve the stability, hydrophilic property, homogeneous property, and endurance property.
Chain Extenders
This is a low molecular weight polyfunctional compounds, reactive with isocyanates and are also known as curing agent. Chain extenders are difunctional glycols, diamines or hydroxyl amines and are use in adhesives, flexible foams, elastomers and RIM systems. The chain-extender reacts with an isocyanate to form a polyurethane or polyurea segment in the polyurethane polymer. Through reactons with excess isocyanate, allophonates and biuret can be formed, transforming the chain-extender effectively into thermo-reversible cross-linker.
Surfactants
Surfactants are often used to improve the properties of foam as well as non-foam PU polymers. They resemble block polymers of polydimethylsiloxane–polyoxyalkylene, nonylphenol ethoxylates, silicone oils and some other organic compounds. In applications that involve foams, they are applied for the emulsification of liquid components, the regulation of cell sizes and for stabilization of cell structures to guide against collapse as well as against voids at the sub surface.
Plasticisers
Plasticisers are used to reduce material hardness. Plasticisers or dispersants are additives that decrease the plasticity or decrease the viscosity of a material. These are the substances which are added in order to alter their physical properties. These are either liquids with low volatility or solids. They decrease the attraction between polymer chains to make them more flexible.
Pigments
Pigments are used to produce coloured PU materials, especially for aesthetic purposes. A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption.
Flame retardants
The term flame retardants subsume a diverse group of chemicals which are added to manufacture materials, such as plastics and textiles, and surface finishes and coatings to prevent or slow the further development of ignition by a variety of different physical and chemical methods. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or (particularly for textiles) applied as a topical finish.
Filler
Filler are used to minimize cost and to improve the material properties, such as tensile strength, toughness, heat resistance, color, clarity and stiffness. Filler materials are particles added to resin or binders (plastics, composites, concrete) that can improve specific properties, make the product cheaper, or a mixture of both.
PU Thickener
Thickeners are included in coating formulations to bring about certain specific required rheological properties. A coating’s rheology influences the properties of the coating during manufacture, storage and application. Present time a new class of thickeners, known as the associative thickeners, used in coatings and, in the meantime, found a variety of applications.
The most popular associative thickeners in waterborne coatings are the HEUR thickeners, also known as PUR (polyurethane) associative thickeners. PUR associative thickeners are a group of synthetic thickeners characterized by a relative low molecular mass (about 10,000–50,000). They permit the formulation of waterborne coatings, with rheological properties virtually identical to those of alkyd resin coatings.
Application of Waterborne Polyurethane
- Surgical garment, hospital drapes, wound dressing and filtration.
- Fire smoke curtains in ships, Cargo wraps
- Special Military, Police Jackets.
- Outerwear for Winter Sports
- Golf Suite, Hats, Gloves.
- Inflatable Tents.
- Sleeping Bags cover.
- Sport footwear linings
- Wood Coating
- Water Proof Paint
Reference:
- George Woods, The ICI Polyurethanes Book, 2nd Edition, 1987, p.197.
- Delebecq, J.-P. Pascault, B. Boutevin and F. O. Ganachaud, On the versatility of urethane/urea bonds: reversibility, blocked isocyanate, and non-isocyanate polyurethane, Chem. Rev., 2012, 113, 80–118,
- Ionescu, Chemistry and technology of polyols for polyurethanes, Rapra Technology, Shrewsbury, UK, Polymer International, 2007, vol. 56
- Gurunathan, S. Mohanty and S. K. Nayak, Effect of reactive organoclay on physicochemical properties of vegetable oil-based waterborne polyurethane nanocomposites, RSC Adv., 2015, 5, 11524–11533
- Jaudouin, J. J. Robin, J. M. Lopez-Cuesta, D. Perrin and C. Imbert, Ionomer-based polyurethanes: a comparative study of properties and applications, Polym. Int., 2012, 61, 495–510
- Gite, P. Mahulikar and D. Hundiwale, Preparation and properties of polyurethane coatings based on acrylic polyols and trimer of isophorone diisocyanate, Prog. Org. Coat., 2010, 68, 307–312
- Lei, Z. Xia, C. Ou, L. Zhang and L. Zhong, Effects of crosslinking on adhesion behavior of waterborne polyurethane ink binder, Prog. Org. Coat., 2015, 88, 155–163
- Lee, J. H. Choi, I.-K. Hong and J. W. Lee, Curing behavior of polyurethane as a binder for polymer-bonded explosives, J. Ind. Eng. Chem., 2015, 21, 980–985
- Zhang, X. Y. Zhang, J. B. Dai and W. H. Li, Synthesis and characterization of yellow waterbornepolyurethane using a diol colorant as extender, Chin. Chem. Lett., 2010, 21, 143–145
- Fang, X. Zhou, Q. Yu, S. Liu, D. Guo and R. Yu, Synthesis and characterization of low crystalline waterborne polyurethane for potential application in water-based ink binder, Prog. Org. Coat., 2014, 77, 61–71
- Fangcq and S. Zhous, The effect of additives to the polyurethane water-based ink, Res. J. Chem. Environ., 2011, 15, 377–379
- Li, X. Zhang, Z. Liu, W. Li and J. Dai, Studies on waterborne polyurethanes based on new medium length fluorinated diols, J. Fluorine Chem., 2015, 175, 12–17
- Fan, W. Du, Z. Li, N. Dan and J. Huang, Abrasion resistance of waterborne polyurethane films incorporated with PU/silica hybrids, Prog. Org. Coat., 2015, 86, 125–133
- Fu, Z. Zheng, Z. Yang, Y. Chen and L. Shen, A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material, Prog. Org. Coat., 2014, 77, 53–60
- Zheng, J. Luo, D. Zhou, T. Shen, H. Li, L. Liang and M. Lu, Preparation and properties of non-ionic polyurethane surfactants, Colloids Surf., A, 2010, 363, 16–21
- https://www.pcimag.com/articles/86154-new-associative-thickeners-and-their-use-in-waterborne-and-high-solids-coatings
https://coatings.specialchem.com/tech-library/article/rheology-in-paints-and-coatings
Author – Brijesh Kr. Bajpai

