By:
Dr. Ruma Chakrabarti, Business Head India, Centexbel
Mr. A. Vignesh, Director AAE
Textile fibres do not appear perfectly white due to the presence of certain coloured impurities. Whitening with optical brigthners or fluorescent brightening agents can eliminate the yellowish hue of the materials. The optical brightening agents counteract the yellowness of the fabric by increasing the reflection of the blue light rays. They convert invisible short wave ultraviolet rays of sunlight into visible blue light and effectively neutralize the pale yellow or cream colour of the white materials; hence a degree of whiteness, which is comparatively more intense, is achieved. The greatest use of brighteners is in detergents, and almost every commercial detergent contains one or more brighteners, in the proportion of 0.05-0.3%
The fluorescent substance to act as a whitening agent must essentially emit light in the blue region so that it effectively neutralizes the normal pale yellow or cream colour of the so-called white materials. OBA is based on the addition of light, whereas the bluing method achieves its white effect through the removal of light.
The optical brighteners should fulfill the following two requirements:
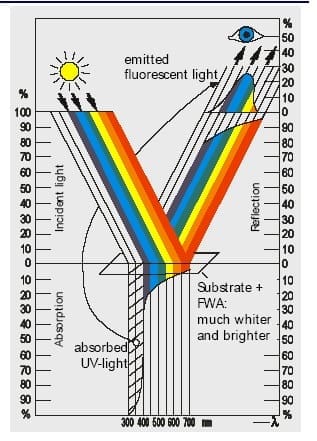
- It should be optically colourless on the substrate
- It should not absorb in the visible part of the spectrum.
The overall effect given by a whitening agent depends on a number of factors:
- The intrinsic effectiveness of the compound as an OBA.
- Its spectral absorption and emission characteristics.
- The ultra-violet content of the viewing light.
Factors Influencing the functions of Optical Whiteners:
Optical brighteners are applied to substrate a s a separate after treatment process or are incorporated into the bleaching and finishing baths. Since the fluorescent brightening agents behave like dyestuffs, their efficiency and effectiveness are influenced by various factors that are important in application.
Substrate: The brightening effect is dependent on the nature of the substrate. For e.g. A very strong reflectance is observed whitened cotton, but is weaker in viscose and wool. In the absence of sufficient affinity of brighteners, the application results in yellow to green colour yield.
Saturation: There is a saturation limit for each optical whitening agent. Above certain concentration on the fibre a yellow colour is superimposed on the fluorescence resulting in decrease in whiteness.
Method of Application: The saturation limit of an optical brightening agent however is also dependent on the method of application to the substrate. Usually exhaust application process gives higher whiteness value than it does when applied by padding technique for a given amount of whitener.
Time: Generally optical brightening agents have high rate of exhaustion on the substrate and therefore great care is to be taken to avoid unlevel application. Slow exhaustion rate and increased migration time is necessary to produce level whiteness on the fabric.
Temperature: The optimum temperatures of optical brightening agents on cellulosic fibres are usually between 40 and 60 C and further rise in temperature tend to lower the exhaustion.
pH : The chemical stability, solubility and affinity of optical brightening agents depend on effective pH value in solution.
Application of Optical Brightening Agents:
- Laundry detergents (to replace whitening agents removed during washing and to make the clothes appear cleaner.) – detergents may contain up to 0.2% whitening agents,
- Paper, especially high brightness papers, resulting in their strongly fluorescent appearance under UV illumination. Paper brightness is typically measured at 457nm,well within the fluorescent activity range of brighteners. Paper used forbanknotes does not contain optical brighteners, so a common method for detecting counterfeit notes is to check for fluorescence.
- Cosmetics: The brightener can not only increase the luminance and sparkle of the hair, but can also correct dull, yellowish discoloration without darkening the hair. Some advanced face and eye powders contain optical brightener microspheres that brighten shadowed or dark areas of the skin, such as “tired eyes”.
- Fabrics may at times contain 0.5% OBAs. A side effect of the presence of OBA is to make the treated fabrics more visible with Night Vision Devices than non-treated ones (the fluorescence caused by optical brighteners can easily be seen with an ordinary black light). This may or may not be desirable for military or other applications
Chemistry of Optical Brighteners
Optical brightening agents are generally based on compounds of stilbene, coumarins, pyrazolins, naphthalimides, naphthooxazoles etc.
- Carbocyclic compounds: Whiteners in this class have a high degree of whiteness and specially suitable for use in detergents, plastics and Polyester substrates.
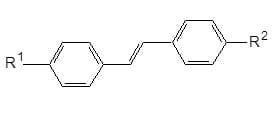
Stilbenes: These group consists mainly of non-ionic compounds, with general formula as shown in (1) with an extended system of conjugated double bonds. They are chiefly suitable for whitening of polyesters, and also polyamides, cellulose acetates and polyvinyl chloride.
(1) Distyryl – Arenes: The non ionic class of distyryl-arenes are excellent whiteners for whitening polyamides, cellulose acetates and plastics with a general formula
(2) The anionic distyryl arenes containing sulphonic acid groups are relatively high hypochlorite stable whiteners, which exhaust on to cotton and polyamides when used in detergents. A further advantage of these compounds is that in effluents the ethylene bridge is degraded in the presence of light and oxygen leading to the formation of low molecular weight sulpho-carboxylic acids. Cationic products serve for whitening cellulose, polyacrylonitrile and polyamides. They are especially useful for application in detergent in the presence of cationic softeners.

Heterocyclic Substituted stilbenes, styrenes and ethylenes : The heterocyclic groups intensifies the fluorescence of conjugated systems and shifts the fluorescence maximum to a longer wavelength.
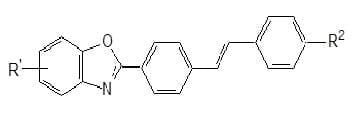
Stilbenyl benzoxazoles: This class consists of high light fastness whiteners for polyester. The non-ionic stibenyl benzoxazoles
(Fig. 3) are suitable for application by the HT or Thermosol Process. They are also suitable for synthetic fibres such as polyamides or cellulose acetate. Anionic stilbenyl benzooxazoles with sulphonic acid groups are whiteners fast to peroxide and chlorine. They are suitable for cotton and polyamides, and for incorporation in detergents ( Fig. 4).
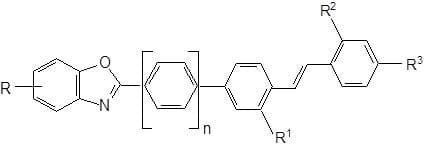
Styryl heteroarenes: The absorption and also the fluorescence of styryl heteroarenes are hypsochromically shifted with respect to those of analogous stilbenyl heteroarenes. They are less efficient and provide whiteness of lower intensity due to their lower molar and specific extinctions.
The nonionic styryl heteroarenes with general structure 5 are suitable for fluorescent whitening of synthetic fibres such as polyesters, polyamides and cellulose acetate.
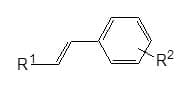
Ionic Styryl Heteroarenes: only 4-naphtho[1,2-d]triazol-2-yl} -a – (benzoxazol-2-yl)styryls with one or two sulphonic groups are known as anionic fluorescent whitening agents, and cationic fluorescent agents of this group have the general formula :
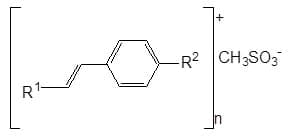
Heteroarenes with one or two ethylene bridges:
- Heteroaryl ethylenes and butadienes: The known whiteners in this class are mainly nonionic. The 5-methyl-benzoxazol-2-yl ring plays an important role in whitening agents for polyester. The polyester whitener based on the aforementioned ring has a very good light fastness and also is easy to apply by both HT and thermosol methods, and as it sublimes easily can also be used in transfer printing.
- Non-ionic heteroarenes with two ethylene bridges: These are high-yield polyester whiteners, also suited for whitening polyamides, cellulose acetates and plastics such as polyvinyl chloride and polyolefins. Their synthesis is very intricate, and the properties are comparable to that of carbocyclic distyryl-arenes.
- Anionic heteroarenes with two ethylene bridges: These compounds contain sulphonic acid groups and are high yield whiteners for cotton and polyamide, and are suitable for application in detergents, textile and paper.
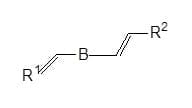
Triazinylaminostilbenes: 1,3,5,-Triazynyl derivatives of 4,4’- diaminostilbene –2-2’-disulphonic acid are the most important class of whiteners in terms of volume. Triazinylaminostilbenes are used for whitening cotton, resin finished textiles, regenerated cellulose fibres and polyamides. They are also used in detergents to whiten textile and paper.
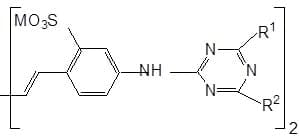
Heterocycles :
Furans and Benzofurans: Furans and benzofurans are usefulbuilding blocks in combination with further cabocyclic and or heterocyclic systems for the synthesis of conjugated fluorescent compounds. The most important classes are the phenylfuranyl and benzofuranyl-benzimidazoles, which are characterized by their high degree of whiteness, good light fastness and chlorite stability.
Benzofurans linked to carbocyclic group: Sulphonated derivative of the compound with general structure (). Sulphonated derivatives of the above are used as whiteners for cotton and polyamides.
Benzodifurans of the given structure with cyano or carboxylic ester groups have been suggested as whiteners for polyester, polystyrene and polyvinyl chloride
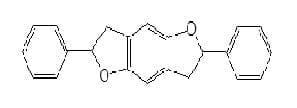
Heterocyclic substituted furans : non ionic products of this are used as whiteners for polyester and polyacrylonitrile.
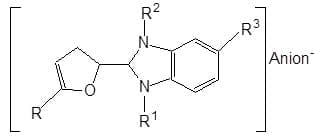
Cationic heterocyclic substituted furans are used to whiten polyacrylonitrile and cellulose acetate.
Heterocyclic substituted benzofuran: Non-ionic compounds of this group are used as whiteners for polyester, polyamides, cellulose acetates and polyvinyl chloride.
Azoles : Mainly non-ionic whiteners have been obtained from this class of compounds. The benzooxazole ring is easy to synthesize and has a good to outstanding light fastness property on polyester substrate.
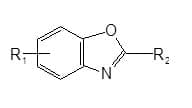
Cationic azoles with the general formula as given below are used to whiten acrylic fibres.
Coumarins: The coumarin ring system substituted in the 3-position by (hetero) aromatic residues or electron-attracting substituents is characterized by its high light stability.
Non-ionic Coumarins: These serve in particular for whitening polyester, but also for polyamides, cellulose acetate, polyolefins and polyvinyl chloride.
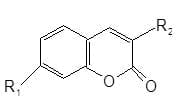
Water soluble Coumarins: Cationic coumarins are used to whiten polyacrylonitrile and cellulose acetate, and are characterized by possessing good light fastness and chlorite stability. The cationic
residue may either be bound to a heterocyclic ring or to a basic side chain. Fibre-reactive coumarins containing sulphonic acid groups are wash resistant whiteners for cotton and polyamide.
Naphthalimides: The naphthalamide ring system has high stability with good light fastness and chlorite stability, the only disadvantage being low molar extinction coefficient.
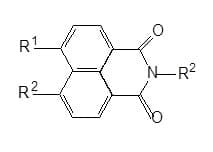
Non-ionic naphthalimides,: N-alkylated naphthalamides are especially used for polyester substrate, but can also be used for whitening polyamide, cellulose acetate, polyolefins and polyvinylchloride.
Water-soluble naphthalimides: Basic and cationic naphthalimides are chlorite-stable whiteners for polyacrylonitrile with good light fastness. They are also recommended for whitening polyester and wool.
Brighteners for Polyester Fibres:
These fibres require disperse dye type fluorescent brighteners due to their hydrophobic nature. The brighteners enter the fibre in a state of molecular dispersion and are held in the fibre by Van-der-Waals forces. The brighteners are usually water insoluble and can be applied in conjunction with a carrier or in HTHP machine. Polyester brighteners belong to the group of compounds such as stilbenyl benzo oxazoles, stilbenyl hetero arenes, heteroaryl ethylenes and butadienesdistyryl arenes, Benzodifurans substituted with cyano or carboxylic ester groups,.non-ionic azoles, naphthalamides etc.
Brighteners for Polyamide fibres: Fluorescent brightener of the acid dyeing coumarin type, 1,3-Diphenyl-2-pyrazolines, and of the stilbene- triazine types are widely used, besides the disperse type of synthetic-fibre brighteners. Water soluble anionic brighteners are applied like acid wool dyes.
Brighteners for Cellulose: For cellulose brighteners with good penetrating properties, and of medium substantivity are required . the brighteners for this category are distyryl arenes, heterocyclic substituted stilbenes, styrenes and ethylenes., benzofurans linked to carboxylic groups.
Ecological Aspect of Optical Brightening Agents:
One of the chemicals used for formulating a Optical brightening agent is called cyanuric chloride, a derivative of 1,3,5 triazine. Cyanuric chloride is used as a precursor and crosslinking agent in sulfonated triazine-stilbene based optical brighterners. It is also classified as “very toxic”, “harmful” and “corrosive” by the EU and has several risk phrases identified with it – including R26 (“very toxic by inhalation”). R26 is a substance which is specifically prohibited by GOTS. Some of the OBAs are allowed, depending on the chemical composition of the individual optical brightener. Like dyestuffs, GOTS allows optical brighteners if they “meet all criteria for the selection of dyes and auxiliaries as defined in chapter 2.4.6, .” Those criteria include the prohibition of all chemicals listed in 2.3.1 and substances which are assigned certain risk phrases “or combinations thereof”.
Some brighteners have been proven to cause allergic skin reactions or eye irritation in sensitive people. The German Textiles Working Group conducted a health assessment of various optical brightening agents following concerns of potential health risks to the public. It was found that there is a general lack of information on toxicity and a need for studies into dermal absorption and the release of these substances from clothes. While it has not been shown to negatively affect health, it has also not been proven safe.
These synthetic chemicals convert UV light wavelengths into visible light, which makes laundered clothes appear whiter (although does not actually affect the cleanliness of the clothing). They’ve been found to be toxic to fish and to cause bacterial mutations. Further, they can cause allergic reactions when exposed to skin that is later exposed to sunlight.OBA’s are known to be toxic to fish and other animal and plant life and have been found to cause mutations in bacteria.
Most OBAs are not readily biodegradable, so chemicals remain in wastewater for long periods of time, negatively affecting water quality and animal and plant life. It is assumed that the substances accumulate in sediment or sludge, leading to high concentrations.
REACH is the new European Union regulation which aims to improve human health and the environment through better and earlier identification of the properties of chemical substances. REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemical substances. REACH contains provisions to reduce the use of what are called “high volume production” chemicals. These are defined as chemicals having annual production and/or importation volumes above 1 million pounds. It is assumed that high volume production is a proxy for high exposure; in addition, large releases of low toxicity substances such as salts do cause environmental harm due to the sheer volume of the substance.
Much of the impact from optical brighteners comes in the form of large releases of low toxicity substances. A number of these optical brighteners are listed as high and low production volume substances and so will be subject to REACH.
In conclusion ObaA is an auxiliary which enhances the aesthetic appeal of white fabrics but they should be used keeping in mind their chemical constitution and hence its ecological and environmental impact.

